SandboxPKA
From Proteopedia
| Line 48: | Line 48: | ||
== '''Structure''' == | == '''Structure''' == | ||
| - | + | ||
| + | All of the protein kinases have a similar bilobal fold, and their key structural features have been well studied <ref>http://www.sciencedirect.com/science/article/pii/S1570963909003069</ref>. | ||
<StructureSection load='1OPK' size='350' side='right' caption='c-Abl tyrosine kinase' scene='SandboxPKA/Abl1/4'> | <StructureSection load='1OPK' size='350' side='right' caption='c-Abl tyrosine kinase' scene='SandboxPKA/Abl1/4'> | ||
| Line 69: | Line 70: | ||
</StructureSection> | </StructureSection> | ||
| + | |||
| + | == Resistance == | ||
| + | |||
Revision as of 15:39, 5 December 2012
Contents |
Introduction
|
The c-Abl protein 1 (ABL1), also known as Abelson kinase, is a non-receptor tyrosine kinase that plays a role in many key processes linked to cell growth and survival such as cytoskeleton remodeling in response to extracellular stimuli, cell motility and adhesion, receptor endocytosis, autophagy, DNA damage response and apoptosis. [1] [2] Activity of c-Abl protein is negatively regulated by its SH3 domain, and deletion of the SH3 domain turns ABL1 into an oncogene. In more than 90% cases, chronic myelogeneous leukemia (CML) is caused by chromosomal abnormality resulting in the formation of a so-called Philadelphia chromosome. It is caused by fusion between Abelson (Abl) tyrosine kinase gene at chromosome 9 and break point cluster (Bcr) gene at chromosome 22, resulting in the chimeric oncogene Bcr-Abl and a constitutively active Bcr-Abl tyrosine kinase.
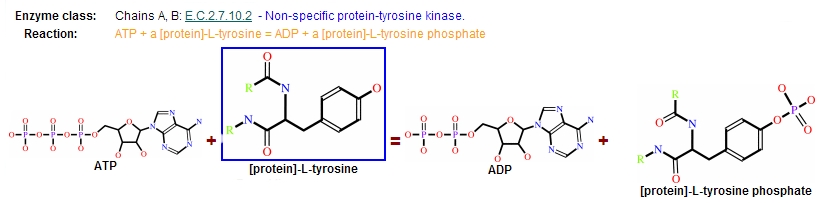
Reaction
Protein kinases are a group of enzymes that possess a catalytic subunit that transfers the gamma (terminal) phosphate from nucleotide triphosphates (often ATP) to one or more amino acid residues in a protein substrate side chain, resulting in a conformational change affecting side protein function.
The enzymes are classified into two broad groups, characterised with respect to substrate specificity:
- Serine/threonine kinases
- Tyrosine specific kinases: c-Abl is included in this group
(Leukemia research 34 (10): 1255–1268. doi:10.1016/j.leukres.2010.04.016. PMID 2053738)
Structure
All of the protein kinases have a similar bilobal fold, and their key structural features have been well studied [3].
| |||||||||||
Catalytic domain
It is responsible of both, ATP binding as well as protein binding.
| |||||||||||