Image:Imatinib bound to receptor.PNG
From Proteopedia
No higher resolution available.
Imatinib_bound_to_receptor.PNG (529 × 221 pixel, file size: 9 KB, MIME type: image/png)
(→Licensing) |
|||
| Line 1: | Line 1: | ||
== Licensing == | == Licensing == | ||
{{subst:No license from license selector|Don't know}} | {{subst:No license from license selector|Don't know}} | ||
| + | |||
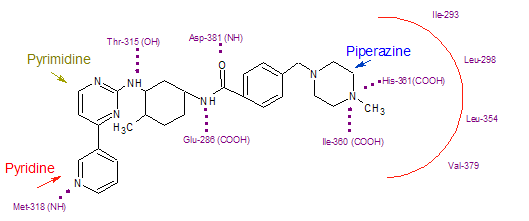
| + | Imatinib binds to Abl domain via six hydrogen bond interactions. This stabilizes the imatinib Bcr-Abl complex and prevents ATP from reaching its binding site.[3][7][9] The hydrogen bonds involve the pyridine-N and backbone-NH of Met-318, the aminopyrimidine and side chain hydroxyl of Thr-315, the amide-NH and side chain carboxylate of Glu-285, the carbonyl and backbone-NH of Asp-381, the protonated methylpiperazine with the backbone-carbonyl atoms of Ile-360 and His-361. Additionally, a number of van der Waals interactions contribute to binding.[7] A hydrophobic pocket is formed by amino acid residues Ile-293, Leu-298, Leu-354 and Val-379 around the phenyl ring adjacent to the piperazinyl-methyl group of imatinib.[9] At the time of its discovery, in the absence of structural information, no clear explanation for the impressive selectivity of imatinib could be found | ||
Revision as of 12:27, 10 December 2012
Licensing
{{subst:No license from license selector|Don't know}}
Imatinib binds to Abl domain via six hydrogen bond interactions. This stabilizes the imatinib Bcr-Abl complex and prevents ATP from reaching its binding site.[3][7][9] The hydrogen bonds involve the pyridine-N and backbone-NH of Met-318, the aminopyrimidine and side chain hydroxyl of Thr-315, the amide-NH and side chain carboxylate of Glu-285, the carbonyl and backbone-NH of Asp-381, the protonated methylpiperazine with the backbone-carbonyl atoms of Ile-360 and His-361. Additionally, a number of van der Waals interactions contribute to binding.[7] A hydrophobic pocket is formed by amino acid residues Ile-293, Leu-298, Leu-354 and Val-379 around the phenyl ring adjacent to the piperazinyl-methyl group of imatinib.[9] At the time of its discovery, in the absence of structural information, no clear explanation for the impressive selectivity of imatinib could be found
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 18:24, 9 December 2012 | Cristina Murga (Talk | contribs) | 529×221 | 9 KB |
- Edit this file using an external application
See the setup instructions for more information.
Links
The following pages link to this file:
