Heidi Hu/Sandbox 2
From Proteopedia
| Line 10: | Line 10: | ||
[http://en.wikipedia.org/wiki/Helicobacter_pylori ''Helicobacter pylori''] is a pathogenic bacterium that colonizes the human gastric mucosa, which can cause peptic ulcers and has been linked to stomach cancers.<ref>PMID:9146793</ref> [http://en.wikipedia.org/wiki/Helicobacter_pylori ''H. pylori''] requires the activity of nickel-dependent enzymes, [NiFe]Hydrogenase and urease, to survive in the acidic environment of the stomach.<ref>PMID:2050411</ref><ref>PMID:8063376</ref><ref>PMID:12459589</ref> Thus nickel is an important nutrient for H. pylori. | [http://en.wikipedia.org/wiki/Helicobacter_pylori ''Helicobacter pylori''] is a pathogenic bacterium that colonizes the human gastric mucosa, which can cause peptic ulcers and has been linked to stomach cancers.<ref>PMID:9146793</ref> [http://en.wikipedia.org/wiki/Helicobacter_pylori ''H. pylori''] requires the activity of nickel-dependent enzymes, [NiFe]Hydrogenase and urease, to survive in the acidic environment of the stomach.<ref>PMID:2050411</ref><ref>PMID:8063376</ref><ref>PMID:12459589</ref> Thus nickel is an important nutrient for H. pylori. | ||
| - | HypA is a nickel metallochaperone normally associated with the maturation of [NiFe]hydrogenase. | + | HypA is a nickel metallochaperone normally associated with the maturation of [NiFe]hydrogenase. In H. pylori, however, it is also require for the full activation of urease, despite the presence of the urease-specific Ni-chaperone, UreE. |
Heavy metals such as iron, nickel, copper, and zinc are important cofactors for the functions of many different metalloenzymes. High levels of these heavy metals can also cause damage to cellular components, therefore intracellular levels of metals are tightly regulated within the cell. One of the ways that bacteria can regulate intracellular metal levels is by increasing the amount of metal efflux proteins. CsoR and RcnR are members of a large family of metal-responsive DNA-binding proteins, both of which regulate the transcription of metal-specific efflux proteins. CsoR is only responsive to the binding of Cu(I); whereas RcnR is only responsive to the binding of Ni(II) or Co(II). | Heavy metals such as iron, nickel, copper, and zinc are important cofactors for the functions of many different metalloenzymes. High levels of these heavy metals can also cause damage to cellular components, therefore intracellular levels of metals are tightly regulated within the cell. One of the ways that bacteria can regulate intracellular metal levels is by increasing the amount of metal efflux proteins. CsoR and RcnR are members of a large family of metal-responsive DNA-binding proteins, both of which regulate the transcription of metal-specific efflux proteins. CsoR is only responsive to the binding of Cu(I); whereas RcnR is only responsive to the binding of Ni(II) or Co(II). | ||
Revision as of 00:10, 12 December 2012
|
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground.
Contents |
Introduction
Helicobacter pylori is a pathogenic bacterium that colonizes the human gastric mucosa, which can cause peptic ulcers and has been linked to stomach cancers.[1] H. pylori requires the activity of nickel-dependent enzymes, [NiFe]Hydrogenase and urease, to survive in the acidic environment of the stomach.[2][3][4] Thus nickel is an important nutrient for H. pylori.
HypA is a nickel metallochaperone normally associated with the maturation of [NiFe]hydrogenase. In H. pylori, however, it is also require for the full activation of urease, despite the presence of the urease-specific Ni-chaperone, UreE.
Heavy metals such as iron, nickel, copper, and zinc are important cofactors for the functions of many different metalloenzymes. High levels of these heavy metals can also cause damage to cellular components, therefore intracellular levels of metals are tightly regulated within the cell. One of the ways that bacteria can regulate intracellular metal levels is by increasing the amount of metal efflux proteins. CsoR and RcnR are members of a large family of metal-responsive DNA-binding proteins, both of which regulate the transcription of metal-specific efflux proteins. CsoR is only responsive to the binding of Cu(I); whereas RcnR is only responsive to the binding of Ni(II) or Co(II).
RcnR and CsoR
In Escherichia coli apo-RcnR blocks the transcription of nickel and cobalt efflux protein RcnA by binding to its promoter region. Although no crystal structures of RcnR is available on the PDB, the Cu(I)-bound CsoR crystal structure from Mycobacterium tuberculosis is available and RcnR is predicted to share a similar fold to CsoR. Upon Ni(II)- or Co(II)-binding, RcnR is released from the DNA allowing the transcription of RcnA facilitating the efflux of Ni(II) and Co(II).[5] CsoR has been characterized in Bacillus subtilis[6] and M. tuberculosis[7] to release from the promoter regions of copper-efflux operons upon binding of Cu(I). The analogous functions of CsoR and RcnR in addition to local sequence similarity (25% identical, 56% similar) suggests a conserved mode of function in this family of metal-responsive DNA-binding proteins.[8]
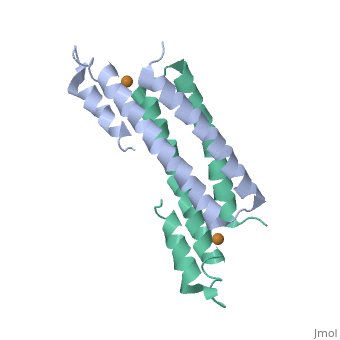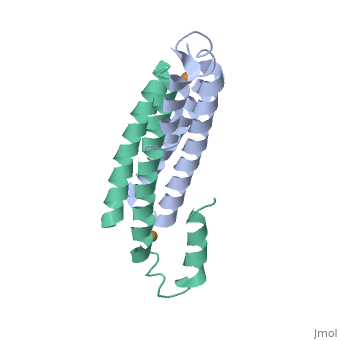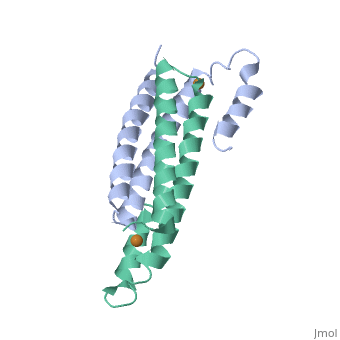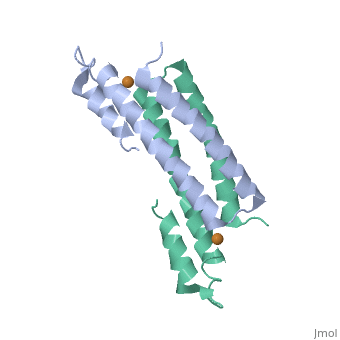
CsoR Structure
binds one Cu(I) per monomer. The protein forms a dimer of dimers with a in the tetrameric interface. Each of a dimer unit. Where one monomer is bound to Cu(I) by His61 and Cys65, the other monomer is bound to the metal by Cys35.[9] E. coli RcnR is also tetrameric and has the same protein-to-metal stochiometry.
Research Interests
The mechanism of DNA binding of the CsoR/RcnR family of metal-responsive transcriptional regulators is still unknown. Additionally, RcnR has an added level of complexity because it is reponsive to both Ni(II) and Co(II) binding. The Maroney Lab at the University of Massachusetts Amherst is interested in the conformational changes of RcnR induced by DNA-, Ni(II)-, and Co(II)-binding.
3D structures of copper homeostasis protein
References
- ↑ Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1997 Apr;11 Suppl 1:71-88. PMID:9146793
- ↑ Eaton KA, Brooks CL, Morgan DR, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991 Jul;59(7):2470-5. PMID:2050411
- ↑ Eaton KA, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994 Sep;62(9):3604-7. PMID:8063376
- ↑ Olson JW, Maier RJ. Molecular hydrogen as an energy source for Helicobacter pylori. Science. 2002 Nov 29;298(5599):1788-90. PMID:12459589 doi:10.1126/science.1077123
- ↑ Iwig JS, Chivers PT. Coordinating intracellular nickel-metal-site structure-function relationships and the NikR and RcnR repressors. Nat Prod Rep. 2010 May;27(5):658-67. Epub 2010 Mar 5. PMID:20442957 doi:10.1039/b906683g
- ↑ Smaldone GT, Helmann JD. CsoR regulates the copper efflux operon copZA in Bacillus subtilis. Microbiology. 2007 Dec;153(Pt 12):4123-8. PMID:18048925 doi:10.1099/mic.0.2007/011742-0
- ↑ Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007 Jan;3(1):60-8. Epub 2006 Dec 3. PMID:17143269 doi:http://dx.doi.org/10.1038/nchembio844
- ↑ Iwig JS, Leitch S, Herbst RW, Maroney MJ, Chivers PT. Ni(II) and Co(II) sensing by Escherichia coli RcnR. J Am Chem Soc. 2008 Jun 18;130(24):7592-606. Epub 2008 May 28. PMID:18505253 doi:10.1021/ja710067d
- ↑ Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007 Jan;3(1):60-8. Epub 2006 Dec 3. PMID:17143269 doi:http://dx.doi.org/10.1038/nchembio844