2axi
From Proteopedia
(New page: 200px<br /> <applet load="2axi" size="450" color="white" frame="true" align="right" spinBox="true" caption="2axi, resolution 1.40Å" /> '''HDM2 in complex wit...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:2axi.gif|left|200px]]<br /> | + | [[Image:2axi.gif|left|200px]]<br /><applet load="2axi" size="350" color="white" frame="true" align="right" spinBox="true" |
| - | <applet load="2axi" size=" | + | |
caption="2axi, resolution 1.40Å" /> | caption="2axi, resolution 1.40Å" /> | ||
'''HDM2 in complex with a beta-hairpin'''<br /> | '''HDM2 in complex with a beta-hairpin'''<br /> | ||
==Overview== | ==Overview== | ||
| - | Inhibitors of the interaction between the p53 tumor-suppressor protein and | + | Inhibitors of the interaction between the p53 tumor-suppressor protein and its natural human inhibitor HDM2 are attractive as potential anticancer agents. In earlier work we explored designing beta-hairpin peptidomimetics of the alpha-helical epitope on p53 that would bind tightly to the p53-binding site on HDM2. The beta-hairpin is used as a scaffold to display energetically hot residues in an optimal array for interaction with HDM2. The initial lead beta-hairpin mimetic, with a weak inhibitory activity (IC(50)=125 microM), was optimized to afford cyclo-(L-Pro-Phe-Glu-6ClTrp-Leu-Asp-Trp-Glu-Phe-D-Pro) (where 6ClTrp=L-6-chlorotryptophan), which has an affinity almost 1,000 times higher (IC(50)=140 nM). In this work, insights into the origins of this affinity maturation based on structure-activity studies and an X-ray crystal structure of the inhibitor/HDM2(residues 17-125) complex at 1.4 A resolution are described. The crystal structure confirms the beta-hairpin conformation of the bound ligand, and also reveals that a significant component of the affinity increase arises through new aromatic/aromatic stacking interactions between side chains around the hairpin and groups on the surface of HDM2. |
==About this Structure== | ==About this Structure== | ||
| - | 2AXI is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens] with SO4 and MPO as [http://en.wikipedia.org/wiki/ligands ligands]. Full crystallographic information is available from [http:// | + | 2AXI is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens] with <scene name='pdbligand=SO4:'>SO4</scene> and <scene name='pdbligand=MPO:'>MPO</scene> as [http://en.wikipedia.org/wiki/ligands ligands]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2AXI OCA]. |
==Reference== | ==Reference== | ||
| Line 15: | Line 14: | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
[[Category: Fasan, R.]] | [[Category: Fasan, R.]] | ||
| - | [[Category: Gruetter, M | + | [[Category: Gruetter, M G.]] |
| - | [[Category: Mittl, P | + | [[Category: Mittl, P R.E.]] |
[[Category: Robinson, J.]] | [[Category: Robinson, J.]] | ||
[[Category: MPO]] | [[Category: MPO]] | ||
| Line 24: | Line 23: | ||
[[Category: protein-protein interactions]] | [[Category: protein-protein interactions]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 16:32:01 2008'' |
Revision as of 14:32, 21 February 2008
|
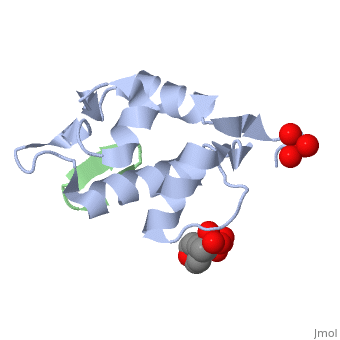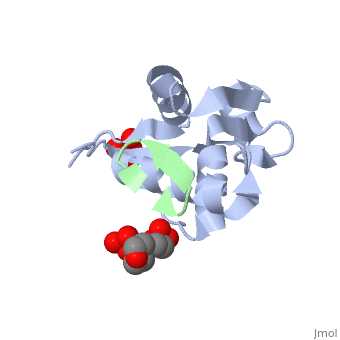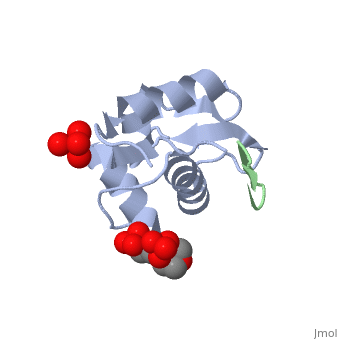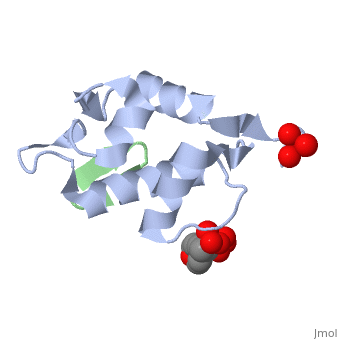
HDM2 in complex with a beta-hairpin
Overview
Inhibitors of the interaction between the p53 tumor-suppressor protein and its natural human inhibitor HDM2 are attractive as potential anticancer agents. In earlier work we explored designing beta-hairpin peptidomimetics of the alpha-helical epitope on p53 that would bind tightly to the p53-binding site on HDM2. The beta-hairpin is used as a scaffold to display energetically hot residues in an optimal array for interaction with HDM2. The initial lead beta-hairpin mimetic, with a weak inhibitory activity (IC(50)=125 microM), was optimized to afford cyclo-(L-Pro-Phe-Glu-6ClTrp-Leu-Asp-Trp-Glu-Phe-D-Pro) (where 6ClTrp=L-6-chlorotryptophan), which has an affinity almost 1,000 times higher (IC(50)=140 nM). In this work, insights into the origins of this affinity maturation based on structure-activity studies and an X-ray crystal structure of the inhibitor/HDM2(residues 17-125) complex at 1.4 A resolution are described. The crystal structure confirms the beta-hairpin conformation of the bound ligand, and also reveals that a significant component of the affinity increase arises through new aromatic/aromatic stacking interactions between side chains around the hairpin and groups on the surface of HDM2.
About this Structure
2AXI is a Single protein structure of sequence from Homo sapiens with and as ligands. Full crystallographic information is available from OCA.
Reference
Structure-activity studies in a family of beta-hairpin protein epitope mimetic inhibitors of the p53-HDM2 protein-protein interaction., Fasan R, Dias RL, Moehle K, Zerbe O, Obrecht D, Mittl PR, Grutter MG, Robinson JA, Chembiochem. 2006 Mar;7(3):515-26. PMID:16511824
Page seeded by OCA on Thu Feb 21 16:32:01 2008