8-Oxoguanine Glycosylase
From Proteopedia
(Difference between revisions)
| Line 26: | Line 26: | ||
Reactive oxygen species (ROS), such as hydrogen peroxide, superoxide and hydroxyl radicals, has been linked to chemical carcinogenesis[1]. Unfortunately, ROS are by-products of aerobic respiration and inflammatory responses, and can also be generated by exposure to ionizing radiation and other free radical generating agents; which are inevitable. ROS can escape mitochondria and attack cellular genome, which results in numerous genotoxic adducts and DNA strand breaks[2]. | Reactive oxygen species (ROS), such as hydrogen peroxide, superoxide and hydroxyl radicals, has been linked to chemical carcinogenesis[1]. Unfortunately, ROS are by-products of aerobic respiration and inflammatory responses, and can also be generated by exposure to ionizing radiation and other free radical generating agents; which are inevitable. ROS can escape mitochondria and attack cellular genome, which results in numerous genotoxic adducts and DNA strand breaks[2]. | ||
DNA bases are susceptible to ROS-mediated oxidation[3]. The low redox potential of guanine makes this base vulnerable to oxidation and leads to a long list of oxidation products[4], among which 7,8-dihydro-8-oxoguanine (also known as 8-oxoG) is one of the most deleterious and frequently formed products (see Fig 1). | DNA bases are susceptible to ROS-mediated oxidation[3]. The low redox potential of guanine makes this base vulnerable to oxidation and leads to a long list of oxidation products[4], among which 7,8-dihydro-8-oxoguanine (also known as 8-oxoG) is one of the most deleterious and frequently formed products (see Fig 1). | ||
| + | {{Clear}} | ||
[[Image:8oxoG and fapyG.gif|thumb|300x200px|upright|''Fig1 Guanine oxidative products: 8-oxoG and fapyG'' [3]]] | [[Image:8oxoG and fapyG.gif|thumb|300x200px|upright|''Fig1 Guanine oxidative products: 8-oxoG and fapyG'' [3]]] | ||
| + | {{Clear}} | ||
[[Image:8oxoG-A.gif|thumb|300x200px|upright|''Fig2 8-oxoG mispairs to A'' [3]]] | [[Image:8oxoG-A.gif|thumb|300x200px|upright|''Fig2 8-oxoG mispairs to A'' [3]]] | ||
| - | + | {{Clear}} | |
The 8-oxoG lesion is particularly deleterious because of its ability to mimic T with its syn conformation, forming a stable 8-oxoG(syn):A(anti) base pair [3](See Fig 2). With this feature, 8-oxoG can efficiently bypass replicative DNA polymerases[5]. Therefore, failure to remove 8-oxoG before replication results in G to T transversion mutations[4](see Fig 3). | The 8-oxoG lesion is particularly deleterious because of its ability to mimic T with its syn conformation, forming a stable 8-oxoG(syn):A(anti) base pair [3](See Fig 2). With this feature, 8-oxoG can efficiently bypass replicative DNA polymerases[5]. Therefore, failure to remove 8-oxoG before replication results in G to T transversion mutations[4](see Fig 3). | ||
| Line 34: | Line 36: | ||
Organisms employ the GO system to avoid the highly mutagenic 8-oxoG. The GO system contains MutT, MutM (also known as Fpg) and MutY enzymes in bacteria[7] and the corresponding MTH1, OGG1 and MUTYH (formerly hMYH) in human[8]. | Organisms employ the GO system to avoid the highly mutagenic 8-oxoG. The GO system contains MutT, MutM (also known as Fpg) and MutY enzymes in bacteria[7] and the corresponding MTH1, OGG1 and MUTYH (formerly hMYH) in human[8]. | ||
MutT/MTH1 hydrolyses 8-oxoGTP and removes it from the nucleotide pool, and thus prevents the incorporation of 8-oxoG into DNA. MutM/OGG1 excises 8-oxoG from the 8-oxoG:C base pairs while MutY/MUTYH removes A from the 8-oxoG:A base pairs in DNA [3]. (see Fig 3). | MutT/MTH1 hydrolyses 8-oxoGTP and removes it from the nucleotide pool, and thus prevents the incorporation of 8-oxoG into DNA. MutM/OGG1 excises 8-oxoG from the 8-oxoG:C base pairs while MutY/MUTYH removes A from the 8-oxoG:A base pairs in DNA [3]. (see Fig 3). | ||
| - | + | {{Clear}} | |
| - | [[Image:Transversion mutation.gif|thumb| | + | [[Image:Transversion mutation.gif|thumb|400x250px|right|''Fig3 human GO system'' [3]]] |
| - | + | {{Clear}} | |
Base excision repair (BER) is the predominate pathway to deal with DNA oxidation damage. | Base excision repair (BER) is the predominate pathway to deal with DNA oxidation damage. | ||
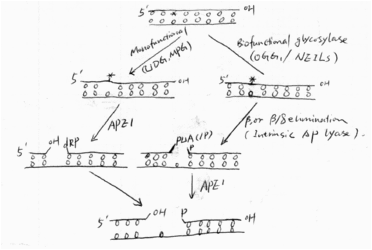
Simply speaking, BER has four steps: first, glycosylases remove the damaged base and leave an AP site; second, the AP site is cleaved by intrinsic lyase activity of glycosylases or an AP endonuclease, followed by some end cleaning processes; third, a DNA polymerase (e.g. Pol) fills the gas with a new nucleotide; and finally, a ligase seals the nick. The figure shows the first two steps of the pathway[9].(See Fig 4) | Simply speaking, BER has four steps: first, glycosylases remove the damaged base and leave an AP site; second, the AP site is cleaved by intrinsic lyase activity of glycosylases or an AP endonuclease, followed by some end cleaning processes; third, a DNA polymerase (e.g. Pol) fills the gas with a new nucleotide; and finally, a ligase seals the nick. The figure shows the first two steps of the pathway[9].(See Fig 4) | ||
| - | [[Image:First 2 steps in BER.gif| | + | {{Clear}} |
| - | + | [[Image:First 2 steps in BER.gif|400x250px|thumb|right|''Fig4 First 2 steps in BER'']] | |
| + | {{Clear}} | ||
===Biological function of OGG1=== | ===Biological function of OGG1=== | ||
OGG1 (8-oxoG glycosylase) is a glycosylase in base excision repair (BER) pathway that particularly deal with guanine oxidative products. It has been shown that hOGG1 is responsible for most o the glycosylase activity against 8-oxoG in human cells[10]. It removes 8-oxoG from double stranded DNA, and thus prevents the potential G to T transversion mutation. | OGG1 (8-oxoG glycosylase) is a glycosylase in base excision repair (BER) pathway that particularly deal with guanine oxidative products. It has been shown that hOGG1 is responsible for most o the glycosylase activity against 8-oxoG in human cells[10]. It removes 8-oxoG from double stranded DNA, and thus prevents the potential G to T transversion mutation. | ||
| Line 75: | Line 78: | ||
===Damage Base Search=== | ===Damage Base Search=== | ||
Based on the structure studies of OGG1 and MutM trapped with DNA, David et.al. proposed an 8-oxo-G lesion search process (shown in fig x)[3]. The 8-oxoG DNA glycosylase moves rapidly along the helix, inserting the probe ligand (for example, Phe 114 in MutM) into the helix to interrogate the base pairs. Intercalation of an amino-acid residue of the enzyme at a normal base pair merely buckles the base pair; however, such a probing event might disrupt an abnormal base pair such as 8-oxoG•C. Such a search process would be extremely fast, so an 8-oxoG base might be missed. However, the research process was proposed to be redundant. When an 8-oxoG encounters hOGG1, it will be expelled from the helix, captured by the ‘exo’ site and passed to the active site. Occasionally expelled undamaged guanine will be captured by ‘exo’ site, but not be processed to the active site; instead, it will be placed back to the helix[3]. (See Fig 5) | Based on the structure studies of OGG1 and MutM trapped with DNA, David et.al. proposed an 8-oxo-G lesion search process (shown in fig x)[3]. The 8-oxoG DNA glycosylase moves rapidly along the helix, inserting the probe ligand (for example, Phe 114 in MutM) into the helix to interrogate the base pairs. Intercalation of an amino-acid residue of the enzyme at a normal base pair merely buckles the base pair; however, such a probing event might disrupt an abnormal base pair such as 8-oxoG•C. Such a search process would be extremely fast, so an 8-oxoG base might be missed. However, the research process was proposed to be redundant. When an 8-oxoG encounters hOGG1, it will be expelled from the helix, captured by the ‘exo’ site and passed to the active site. Occasionally expelled undamaged guanine will be captured by ‘exo’ site, but not be processed to the active site; instead, it will be placed back to the helix[3]. (See Fig 5) | ||
| - | + | {{Clear}} | |
| - | [[Image:HOGG1 searching model.gif|thumb| | + | [[Image:HOGG1 searching model.gif|thumb| 400x250px|center|''Fig5 A model of damage base searching and recognition'' [3]]] |
| - | + | {{Clear}} | |
===‘Base Flip’=== | ===‘Base Flip’=== | ||
The base extrusion (Base flip) from DNA helix to the enzyme active sites has been described as a ‘pinch-push-plug-pull’ motion. First, glycosylase induces bending and distortion of the DNA double helix (pinch). Second, the glycosylase intercalates an amino acid side chain into the DNA helix, to ‘push’ the target base out of the helix. The same or another intercalating amino acid functions as a ‘plug’ to fill the gap left by the extruded base and stabilizes distorted DNA duplex. A ‘pull’ by active-site residues specific for the relevant target base secures it into the damage base recognition pocket [3,20]. | The base extrusion (Base flip) from DNA helix to the enzyme active sites has been described as a ‘pinch-push-plug-pull’ motion. First, glycosylase induces bending and distortion of the DNA double helix (pinch). Second, the glycosylase intercalates an amino acid side chain into the DNA helix, to ‘push’ the target base out of the helix. The same or another intercalating amino acid functions as a ‘plug’ to fill the gap left by the extruded base and stabilizes distorted DNA duplex. A ‘pull’ by active-site residues specific for the relevant target base secures it into the damage base recognition pocket [3,20]. | ||
| Line 129: | Line 132: | ||
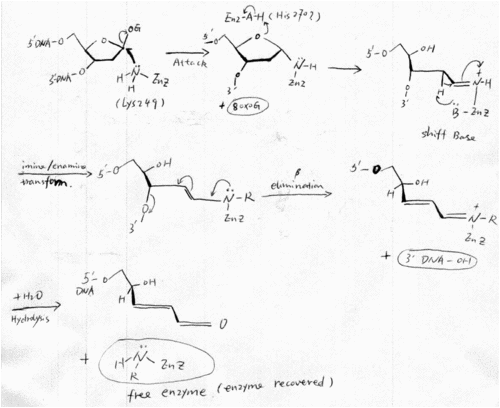
After Nash et.al. cloned hOGG1 in 1996, its catalytic mechanism has been widely researched. Before the resolve of hOGG1 crystal structure, Nash et. al. found that lysine 249 is the critical catalytic amino acid, by using covalent linkage of damage DNA to hOOG1 and Edaman sequencing [22]. This finding well consist with the proposed mechanism of NTH family (hOGG1 was defined as a member of NTH family). And Lysine 249 also seems likely to be the catalytic amino acid that acts as the nucleophile and Shiff base donor in the crystal structure. His270 was proposed to be the amino acid that protonate the oxygen of the deoxyribose ring (See part 2). | After Nash et.al. cloned hOGG1 in 1996, its catalytic mechanism has been widely researched. Before the resolve of hOGG1 crystal structure, Nash et. al. found that lysine 249 is the critical catalytic amino acid, by using covalent linkage of damage DNA to hOOG1 and Edaman sequencing [22]. This finding well consist with the proposed mechanism of NTH family (hOGG1 was defined as a member of NTH family). And Lysine 249 also seems likely to be the catalytic amino acid that acts as the nucleophile and Shiff base donor in the crystal structure. His270 was proposed to be the amino acid that protonate the oxygen of the deoxyribose ring (See part 2). | ||
| - | [[Image:Hogg mechnism.gif|thumb| | + | {{Clear}} |
| - | + | [[Image:Hogg mechnism.gif|thumb|500x700px|center|''Fig6 Catalytic mechanism of hOGG'' [22]] | |
| + | {{Clear}} | ||
===Similar mechanisms of mOGG1=== | ===Similar mechanisms of mOGG1=== | ||
The amino acid sequence of hOGG1 and mOGG1 are very similar, so are their enzymatic activities and substrate specificities. In addition, the reaction mechanism proposed by Zharkov et.al. is also consistent with that of hOGG1 [27]. In their paper that, three possible pathways were proposed.In all the three cases, the ε-amino group of Lys249 attacks at C1’ of 8-oxodG. A proton donor may interact with O8 (A) or a heterocyclic deoxyribose oxygen (B)to initiate nucleophilic attack at C1'. However, Lys249 may carry out a direct SN2 displacement and form an oxycarbenium intermediate (C). After the attack, 8-oxoG is expelled, and a Schiff base is formed between Lys249 and C19. The Schiff base is hydrolyzed to recover the enzyme after DNA backbone cleavage[27] (see Fig x) | The amino acid sequence of hOGG1 and mOGG1 are very similar, so are their enzymatic activities and substrate specificities. In addition, the reaction mechanism proposed by Zharkov et.al. is also consistent with that of hOGG1 [27]. In their paper that, three possible pathways were proposed.In all the three cases, the ε-amino group of Lys249 attacks at C1’ of 8-oxodG. A proton donor may interact with O8 (A) or a heterocyclic deoxyribose oxygen (B)to initiate nucleophilic attack at C1'. However, Lys249 may carry out a direct SN2 displacement and form an oxycarbenium intermediate (C). After the attack, 8-oxoG is expelled, and a Schiff base is formed between Lys249 and C19. The Schiff base is hydrolyzed to recover the enzyme after DNA backbone cleavage[27] (see Fig x) | ||
| - | + | {{Clear}} | |
| - | [[Image:MOGG1 mechanism.gif|thumb| | + | [[Image:MOGG1 mechanism.gif|thumb|400x250px|center|''Three proposed mechanisms of mOGG1'' [27]]] |
| - | + | {{Clear}} | |
===Monofunctional glycosylase mechanism=== | ===Monofunctional glycosylase mechanism=== | ||
The mechanism of monofunctional glycosylase appeared earlier than that of biofunctional glycosylases. The mechanisms of AlkA and UDG are well studied. In the case of these monofunctional glycosylases, the enzymes deliver an activated water molecule to the glycosidic bond of the substrate. The tightly bound water molecule is most likely to perform the <ref>PMID:8347626</ref> nucleophilic attacking to the C1’ of the deoxyribose ring [32]. | The mechanism of monofunctional glycosylase appeared earlier than that of biofunctional glycosylases. The mechanisms of AlkA and UDG are well studied. In the case of these monofunctional glycosylases, the enzymes deliver an activated water molecule to the glycosidic bond of the substrate. The tightly bound water molecule is most likely to perform the <ref>PMID:8347626</ref> nucleophilic attacking to the C1’ of the deoxyribose ring [32]. | ||
Revision as of 11:59, 11 March 2013
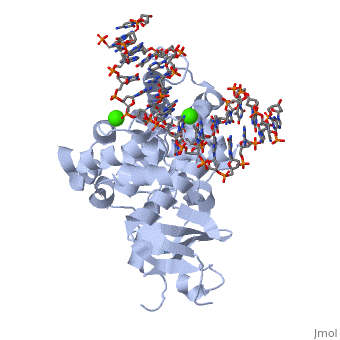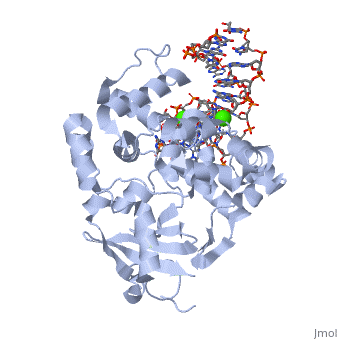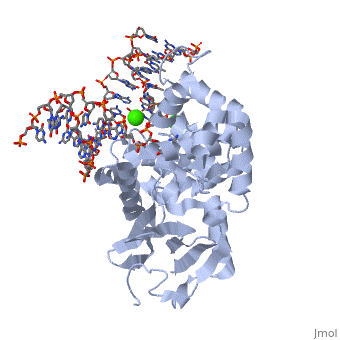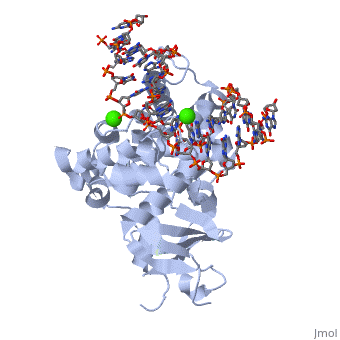
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, Riley Hicks, Michal Harel, David Canner, Jaime Prilusky, Andrea Gorrell
![Fig1 Guanine oxidative products: 8-oxoG and fapyG [3]](/wiki/images/thumb/0/0d/8oxoG_and_fapyG.gif/300px-8oxoG_and_fapyG.gif)
![Fig2 8-oxoG mispairs to A [3]](/wiki/images/thumb/6/6d/8oxoG-A.gif/300px-8oxoG-A.gif)
![Fig3 human GO system [3]](/wiki/images/thumb/e/e5/Transversion_mutation.gif/376px-Transversion_mutation.gif)
![Fig5 A model of damage base searching and recognition [3]](/wiki/images/thumb/2/2c/HOGG1_searching_model.gif/400px-HOGG1_searching_model.gif)
![Three proposed mechanisms of mOGG1 [27]](/wiki/images/thumb/f/fe/MOGG1_mechanism.gif/273px-MOGG1_mechanism.gif)
