Tertiary structure
Chymotrypsin is initially synthesized as a 245 amino acid inactive precursor (a zymogen) termed chymotrypsinogen. Activation of chymotrypsinogen involves proteolytic cleavage at two sites along the chain and removal of two amino acids at each cleavage site. The resultant are shown here (chain 1 = 1-13 in green; chain 2 = 16-146 in red; chain 3 = 149-24 in blue). Note, some amino acids at the temini of these chains are not shown in this representation (e.g. 11-13, 149, ). This is because these residues show too much flexibility in the crystal structures to give X-ray diffraction patterns which would locate them in space.
The three chains are held together by five . Can you identify the specific cys residues linked in each disulfide bond? Why do you think is it very difficult to obtain active chymotrypsin after denaturation and renaturation?
Beta Barrels, Protein Domains and the Active Center
The chymotrypsin molecule is folded into two , each containing six beta strands (orange) arranged as anti-parallel sheets which form a circular structure known as a beta barrel. Rotate the molecule so that you can see down through each of the two beta barrels in turn.
The (Ser-195, His-57 and Asp-102 shown here in spacefill representation), are far apart in the primary sequence but are brought together in a crevice formed between the two beta barrel protein domains.
Colour key:
Alpha Helices,
Beta Strands .
The Active Site Triad
The of chymotrypsin consists of Asp102 positioned close to His 57 and Ser 195. The precise mechanism of action is still debated, but it appears that a hydrogen on the his imidazole ring is transferred to the Asp 102 carboxylate (either via a "charge relay system" or via a "low barrier H-bond"). This shift results in the histidine ring being able to accept the serine 195 hydroxyl hydrogen, forming a very nucleophilic serine alkoxide ion.
Binding of the Substrate
This structure contains a competitive inhibitor, . This is a dipeptide of Leu and Phe (orange), plus a fluorobenzylamide group (red), which is aromatic and bound in the specificity pocket (see next button). In an actual substrate, the peptide bond cleaved would be to the carboxyl side of the aromatic amino acid. In this inhibitor, there are two residues to the amide side, but there is no residue to what would be the carboxyl side. Thus, there is no cleavable bond in this structure.
The Active Site Environment
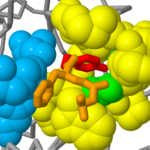
A specific pocket adjacent to the active site triad determines the specificity of the protease (chymotrypsin cleaves adjacent to large aromatic side chains, trypsin adjacent to Lys or Arg residues). In this view, the are shown as spacefilling (yellow) and the three residues which are predominant determinants of this specificity are shown in shades of green. These are amino acids 189, 216 and 226 which line a pocket adjacent to the active site triad. The residues in the catalytic triad are blue, whilst the fluorobenzylamide inhibitor is now shown as a stick representation.
Here, the fluorobenzylamide group (red) of the inhibitor is bound in this pocket. In trypsin and chymotrypsin, residues 216 and 226 are both glycine (lime green), which has a minimal side chain, leaving space so that bulky side chains in the substrate protein can extend into the interior of this pocket. In contrast, in elastase, these residues are Val and Thr, which have side chains that partly fill the pocket so that bulky R groups will not fit into it. In chymotrypsin, residue 189 is a serine (green) and this allows bulky aromatic R groups to interact with the pocket predominantly via van der Waals forces. In trypsin, residue 189 is the negatively-charged Asp, and this allows binding of substrates with positively charged Lys or Arg residues.