Single stranded binding protein
From Proteopedia
| Line 25: | Line 25: | ||
When His-55 is substituted with Leu it decreases binding affinity. All of these residues | When His-55 is substituted with Leu it decreases binding affinity. All of these residues | ||
are found in a hydrophobic region, which is suitable for nucleotide base interactions. | are found in a hydrophobic region, which is suitable for nucleotide base interactions. | ||
| + | |||
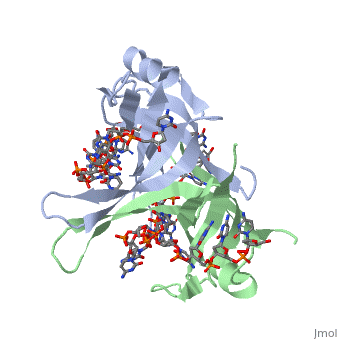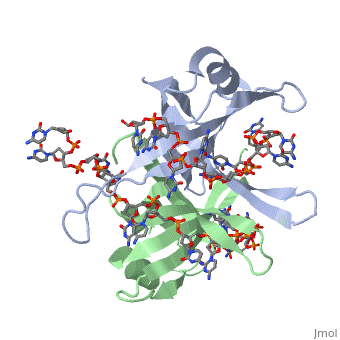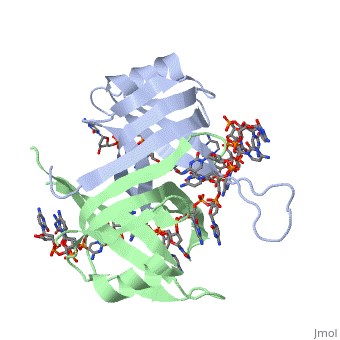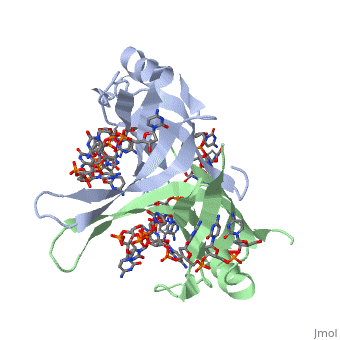
| + | <StructureSection load='2vw9' size='500' side='right' frame='true' caption='Structure of Single Stranded DNA-Binding Protein bound to ssDNA (PDB entry [[2vw9]])' scene=''> | ||
===SSB-Protein Interactions=== | ===SSB-Protein Interactions=== | ||
| - | It is believed that Gly-15 may play an important role in binding the RecA protein. Mutations in Gly-15 have extreme effects on recombinational repair. SSB has also been thought to bind with exonuclease I, DNA polymerase II, and a protein n, which is used to help synthesize RNA primers for the lagging strand. | + | It is believed that Gly-15 may play an important role in binding the RecA protein. Mutations in Gly-15 have |
| - | + | extreme effects on recombinational repair. SSB has also been thought to bind with exonuclease I, DNA polymerase II, | |
| - | + | and a protein n, which is used to help synthesize RNA primers for the lagging strand. | |
</StructureSection> | </StructureSection> | ||
Revision as of 15:00, 1 November 2013
Contents |
Sandbox Single Stranded DNA-Binding Protein (SSB)
| |||||||||||
Binding Interactions in the Active Site
Phe-60 is an important DNA binding site. It has been shown to be the site for cross-linking. Tryptophan and Lysine residues are important in binding as well. “Treatments resulting in modification of arginine, cysteine, or tyrosine residues had no effect on binding of SSB to DNA, whereas modification of either lysine residues (with acetic anhydride) or tryptophan residues (with N-bromosuccinimide) led to complete loss of binding activity” ( Meyer, 348). The two tryptophan residues involved in DNA binding are Try-40 and Try-54, which was determined by mutagenesis. One more binding site was determined by site-specific mutagenesis. When His-55 is substituted with Leu it decreases binding affinity. All of these residues are found in a hydrophobic region, which is suitable for nucleotide base interactions.
| |||||||||||
See Also
References
PMID: 2087220
Proteopedia Page Contributors and Editors (what is this?)
Refayat Ahsen, Rachel Craig, Michal Harel, Alexander Berchansky