Serine/threonine protein kinase
From Proteopedia
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='' size='450' side='right' scene='Journal:JBIC:2/Opening/1' caption='Crystal Structure of Glycogen Synthase Kinase 3ß bound to Anticancer Ruthenium Complex'> | |
'''Serine/threonine protein kinase''' 1 (Chk1) phosphorylates cdc25A, cdc25B and cdc25C. Upon phosphorylation, cdc25 binds adaptor protein and the cell is prevented from entering mitosis.<br /> | '''Serine/threonine protein kinase''' 1 (Chk1) phosphorylates cdc25A, cdc25B and cdc25C. Upon phosphorylation, cdc25 binds adaptor protein and the cell is prevented from entering mitosis.<br /> | ||
| Line 5: | Line 5: | ||
'''Chk6''' called also '''Aurora A''' is critical for the formation of mitotic spindles during cellular mitosis. Chk6 is phosphorylated at residues Thr287 and Thr288.<br /> | '''Chk6''' called also '''Aurora A''' is critical for the formation of mitotic spindles during cellular mitosis. Chk6 is phosphorylated at residues Thr287 and Thr288.<br /> | ||
'''Chk13 (Polo-like kinase 1 or Plk1)''' functions during the M phase of the cell cycle including the regulation of centrosome maturation and spindle assembly. Chk13 binds and phosphorylates proteins which are already phosphorylated on a motif recognized by its POLO-box domain (Pbd) at the C terminal. Human Chk13 contains catalytic domain (residues 13-345) and POLO-box domain (residues 345-603). | '''Chk13 (Polo-like kinase 1 or Plk1)''' functions during the M phase of the cell cycle including the regulation of centrosome maturation and spindle assembly. Chk13 binds and phosphorylates proteins which are already phosphorylated on a motif recognized by its POLO-box domain (Pbd) at the C terminal. Human Chk13 contains catalytic domain (residues 13-345) and POLO-box domain (residues 345-603). | ||
| + | |||
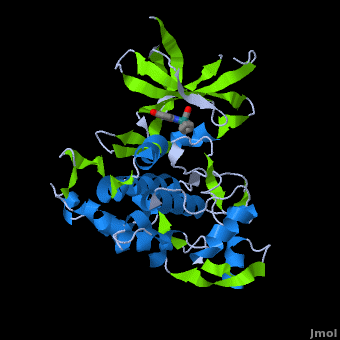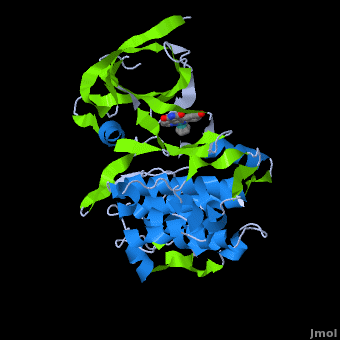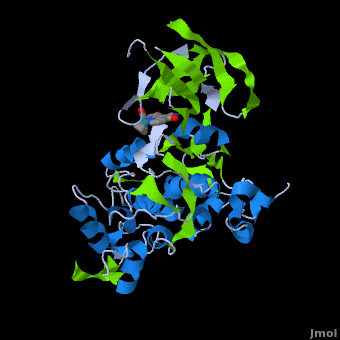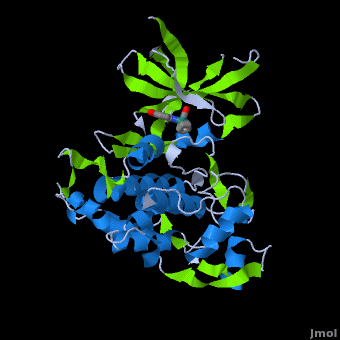
| + | '''Glycogen synthase kinase 3''' (GSK-3) is a serine/threonine protein kinase. GSK-3 is active in a number of intracellular signaling pathways. GSK-3 regulates glycogen synthase as well as other proteins. GSK-3 inhibition is studied as a therapeutic target in diseases like Alzheimer, diabetes, bipolar disorder and some cancers. | ||
| + | |||
| + | '''Structure of Anticancer Ruthenium Half-Sandwich Complex Bound to Glycogen Synthase Kinase 3ß <ref>DOI 10.1007/s00775-010-0699-x</ref>''' | ||
| + | A crystal structure of an <scene name='Journal:JBIC:2/Half_sandwich_complex_no_bonds/1'>organometallic half-sandwich ruthenium complex </scene>bound to the protein kinase glycogen synthase kinase 3ß (GSK-3ß) has been determined and reveals that the inhibitor binds to the <scene name='Journal:JBIC:2/Atp_binding_site2/2'>ATP binding site</scene> via an induced fit mechanism utlizing several <scene name='Journal:JBIC:2/Half_sandwich_complex/3'>hydrogen bonds</scene> and <scene name='Journal:JBIC:2/Half_sandwich_hydrophobic_stic/1'>hydrophobic interactions</scene>. Importantly, the metal is not involved in any direct interaction with the protein kinase but fulfills a purely structural role. The unique, bulky molecular structure of the half-sandwich complex with the CO-ligand oriented perpendicular to the pyridocarbazole heterocycle allows the complex to stretch the whole distance <scene name='Journal:JBIC:2/Half_sandwich_hydrophobic/5'>sandwiched between the faces of the N- and C-terminal lobes</scene> and to interact tightly with <scene name='Journal:JBIC:2/Glycine_rich_loop2/4'>the flexible glycine-rich loop</scene>. Although this complex is a conventional ATP-competitive binder, the unique shape of the complex allows novel interactions with the glycine-rich loop which are crucial for binding potency and selectivity. It can be hypothesized that coordination spheres which present other ligands towards the glycine-rich loop might display completely different protein kinase selectivities. | ||
| + | </StructureSection> | ||
| + | |||
__NOTOC__ | __NOTOC__ | ||
Revision as of 12:07, 3 November 2013
| |||||||||||
3D structures of serine/threonine protein kinase
Updated on 03-November-2013
Chk1
1nvq, 1nvr – hChk1 kinase domain+ peptide + saurosporine – human
1nvs, 1zys - hChk1 kinase domain + peptide + inhibitor
2cgu, 2cgv, 2cgw, 2cgx, 2c3j, 2c3k, 2c3l, 2br1, 2brb, 2brg, 2brh, 2brm, 2brn, 2bro, 2ayp, 2gdo, 2ghg, 2hog, 2ywp, 2hxl, 2hxq, 2hy0, 2r0u, 2e9n, 2e9o, 2e9p, 2e9u, 2e9v, 2qhm, 2qhn, 3f9n, 2wmq, 2wmr, 2wms, 2wmt, 2wmu, 2wmv, 2wmx, 3jvr, 3jvs, 2xey, 2xf0, 2xez, 2x8d, 2x8e, 2x8i, 3ot3, 3ot8, 3pa3, 3pa4, 3pa5, 3nlb - hChk1 kinase domain + inhibitor
Chk2
1gxc – hChk2 phosphothreonine-binding domain + phosphopeptide
2cn5 – hChk2 kinase domain + ADP
2cn8 – hChk2 kinase domain + inhibitor
2w0j, 2w7x, 2wtc, 2wtd, 2xbj, 2xm8, 2xm9, 2yiq, 2yir, 2yit - hChk2 kinase domain + inhibitor
3i6u, 3i6w – hChk2 residues 84-502 (mutant)
Chk6
1muo, 1mq4 – hChk6 kinase domain
1ol6 – hChk6 kinase domain (mutant) + ATP
2wqe – hChk6 kinase domain (mutant) + ADP
2c6d – hChk6 kinase domain (mutant) + ADPNP
2dwb – hChk6 kinase domain + AMPPNP
3daj, 3d14, 3dj5, 3dj6, 3dj7, 3d15, 3d2i, 3d2k – Chk6 kinase domain (mutant) + inhibitor - mouse
2j4z, 2j50, 2np8, 3efw, 2x81, 2x6d, 2x6e, 3myg, 3vap, 4b0g – hChk6 kinase domain + inhibitor
2bmc, 2c6e, 3coh, 3h0y, 3h0z, 3h10, 3fdn, 2wtw, 3lau, 3nrm, 2xne, 2xng, 2xru, 3k5u, 3m11, 3p9j, 3r21, 3r22, 3qbn, 3unz, 3uo4, 3uo5, 3uo6, 3uod, 3uoh, 3uoj, 3uok, 3uol, 3up2, 3up7, 4dhf, 4dea, 4deb, 4ded, 4dee – hChk6 kinase domain (mutant) + inhibitor
Chk6 with phosphorylated Thr 287, Thr288
1ol5, 1ol7 – hChk6 kinase domain + PThr + ADP
2w1c, 2w1d, 2w1e, 2w1f, 2w1g – hChk6 kinase domain + PThr + inhibitor
2wtv – hChk6 kinase domain (mutant) + PThr + inhibitor
3e5a, 3ha6 – hChk6 kinase domain + PThr + inhibitor + targeting protein for XKLP2
Chk13
Chk13 POLO-box domain (Pdb)
1q4o, 2ogq, 3hih, 3p2w, 4h5x – hChk13 Pbd
Chk13 Pbd complex with polypeptide
1umw, 2ojx, 3bzi, 3c5l, 3rq7, 4dfw – hChk13 Pbd + peptide
3hik, 3fvh, 3p2z, 3p34, 3p35, 3p36, 3p37, 3q1i, 4e67, 4e9c, 4e9d, 4hab, 4hy2 – hChk13 Pbd + phosphopeptide
1q4k – hChk13 Pbd (mutant) + phosphopeptide
2v5q – hChk13 Pbd + design ankyrin repeat protein
Chk13 Pbd complex with small molecule inhibitor
4h71, 4hco – hChk13 Pbd + inhibitor
2rku – hChk13 Pbd (mutant) + inhibitor
3db6, 3db8, 3dbc, 3dbd, 3dbe, 3dbf – zfChk13 Pbd (mutant) + inhibitor – zebra fish
Chk13 catalytic domain
2owb – hChk13 catalytic domain
2ou7 – hChk13 catalytic domain + AMPPNP
3d5w – zfChk13 catalytic domain + ADP
3kb7, 2yac, 3thb, 4a4l, 4a4o – hChk13 catalytic domain + inhibitor
3fc2 – hChk13 catalytic domain (mutant) + inhibitor
3d5x – zfChk13 catalytic domain (mutant) + wortmannin
Rac-β hChk
1gzk, 1gzn, 1gzo – Rac-β hChk kinase domain
1p6s - Rac-β hChk pleckstrin homology domain - NMR
1o6k - Rac-β hChk kinase domain + GSK3 peptide + AMPPNP
1o6l - Rac-β hChk kinase domain (mutant) + GSK3 peptide + AMPPNP
Various Chk
1c1y – hChk proto oncogene kinase domain + Ras-related protein Rap
2xiy, 2xiz, 2xj0 - hChk proto oncogene Pim-1 kinase domain
1u5q, 1u5r – rChk Tao2 kinase domain – rat
2cos – Chk Lats2 – mouse – NMR
2xik – hChk 25
3hgk – Chk Pto + effector protein AVRPTOB – Currant tomato
3ori, 3ork, 3orl, 3orm, 3oro, 3orp, 3ort - MtChk kinase domain (mutant) – Mycobacterium tuberculosis
3ouv - MtChk extracellular domain