2psm
From Proteopedia
(New page: 200px<br /><applet load="2psm" size="350" color="white" frame="true" align="right" spinBox="true" caption="2psm, resolution 2.19Å" /> '''Crystal structure of...) |
|||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
| - | Interleukin (IL)-15 is a pleiotropic cytokine that plays a pivotal role in | + | Interleukin (IL)-15 is a pleiotropic cytokine that plays a pivotal role in both innate and adaptive immunity. IL-15 is unique among cytokines due to its participation in a trans signaling mechanism in which IL-15 receptor alpha (IL-15Ralpha) from one subset of cells presents IL-15 to neighboring IL-2Rbeta/gammac-expressing cells. Here we present the crystal structure of IL-15 in complex with the sushi domain of IL-15Ralpha. The structure reveals that the alpha receptor-binding epitope of IL-15 adopts a unique conformation, which, together with amino acid substitutions, permits specific interactions with IL-15Ralpha that account for the exceptionally high affinity of the IL-15.IL-15Ralpha complex. Interestingly, analysis of the topology of IL-15 and IL-15Ralpha at the IL-15.IL-15Ralpha interface suggests that IL-15 should be capable of participating in a cis signaling mechanism similar to that of the related cytokine IL-2. Indeed, we present biochemical data demonstrating that IL-15 is capable of efficiently signaling in cis through IL-15Ralpha and IL-2Rbeta/gammac expressed on the surface of a single cell. Based on our data we propose that cis presentation of IL-15 may be important in certain biological contexts and that flexibility of IL-15Ralpha permits IL-15 and its three receptor components to be assembled identically at the ligand-receptor interface whether IL-15 is presented in cis or trans. Finally, we have gained insights into IL-15.IL-15Ralpha.IL-2Rbeta.gammac quaternary complex assembly through the use of molecular modeling. |
==About this Structure== | ==About this Structure== | ||
| Line 10: | Line 10: | ||
==Reference== | ==Reference== | ||
| - | Crystal | + | Crystal Structure of the interleukin-15.interleukin-15 receptor alpha complex: insights into trans and cis presentation., Olsen SK, Ota N, Kishishita S, Kukimoto-Niino M, Murayama K, Uchiyama H, Toyama M, Terada T, Shirouzu M, Kanagawa O, Yokoyama S, J Biol Chem. 2007 Dec 21;282(51):37191-204. Epub 2007 Oct 18. PMID:[http://ispc.weizmann.ac.il//pmbin/getpm?pmid=17947230 17947230] |
[[Category: Mus musculus]] | [[Category: Mus musculus]] | ||
[[Category: Protein complex]] | [[Category: Protein complex]] | ||
| Line 17: | Line 17: | ||
[[Category: Kukimoto-Niino, M.]] | [[Category: Kukimoto-Niino, M.]] | ||
[[Category: Murayama, K.]] | [[Category: Murayama, K.]] | ||
| - | [[Category: Olsen, S | + | [[Category: Olsen, S K.]] |
[[Category: Ota, N.]] | [[Category: Ota, N.]] | ||
| - | [[Category: RSGI, RIKEN | + | [[Category: RSGI, RIKEN Structural Genomics/Proteomics Initiative.]] |
[[Category: Shirouzu, M.]] | [[Category: Shirouzu, M.]] | ||
[[Category: Terada, T.]] | [[Category: Terada, T.]] | ||
| Line 42: | Line 42: | ||
[[Category: transmembrane]] | [[Category: transmembrane]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 18:32:40 2008'' |
Revision as of 16:32, 21 February 2008
|
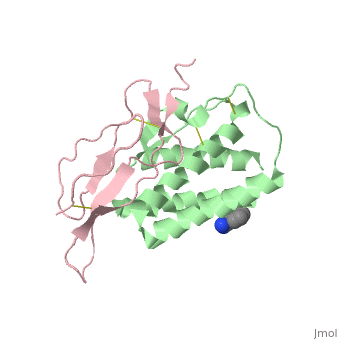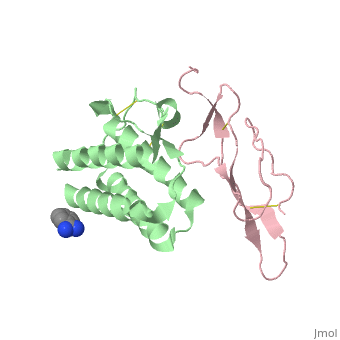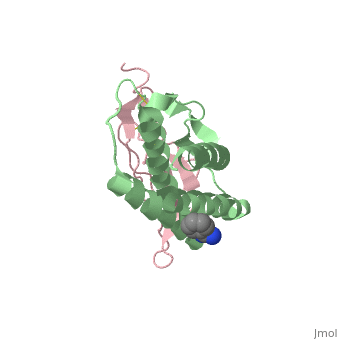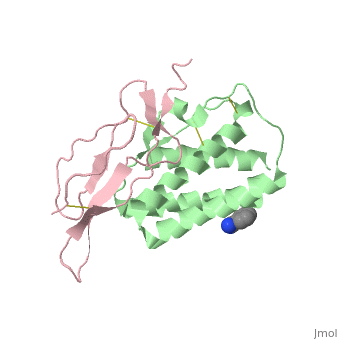
Crystal structure of Interleukin 15 in complex with Interleukin 15 receptor alpha
Overview
Interleukin (IL)-15 is a pleiotropic cytokine that plays a pivotal role in both innate and adaptive immunity. IL-15 is unique among cytokines due to its participation in a trans signaling mechanism in which IL-15 receptor alpha (IL-15Ralpha) from one subset of cells presents IL-15 to neighboring IL-2Rbeta/gammac-expressing cells. Here we present the crystal structure of IL-15 in complex with the sushi domain of IL-15Ralpha. The structure reveals that the alpha receptor-binding epitope of IL-15 adopts a unique conformation, which, together with amino acid substitutions, permits specific interactions with IL-15Ralpha that account for the exceptionally high affinity of the IL-15.IL-15Ralpha complex. Interestingly, analysis of the topology of IL-15 and IL-15Ralpha at the IL-15.IL-15Ralpha interface suggests that IL-15 should be capable of participating in a cis signaling mechanism similar to that of the related cytokine IL-2. Indeed, we present biochemical data demonstrating that IL-15 is capable of efficiently signaling in cis through IL-15Ralpha and IL-2Rbeta/gammac expressed on the surface of a single cell. Based on our data we propose that cis presentation of IL-15 may be important in certain biological contexts and that flexibility of IL-15Ralpha permits IL-15 and its three receptor components to be assembled identically at the ligand-receptor interface whether IL-15 is presented in cis or trans. Finally, we have gained insights into IL-15.IL-15Ralpha.IL-2Rbeta.gammac quaternary complex assembly through the use of molecular modeling.
About this Structure
2PSM is a Protein complex structure of sequences from Mus musculus with as ligand. Full crystallographic information is available from OCA.
Reference
Crystal Structure of the interleukin-15.interleukin-15 receptor alpha complex: insights into trans and cis presentation., Olsen SK, Ota N, Kishishita S, Kukimoto-Niino M, Murayama K, Uchiyama H, Toyama M, Terada T, Shirouzu M, Kanagawa O, Yokoyama S, J Biol Chem. 2007 Dec 21;282(51):37191-204. Epub 2007 Oct 18. PMID:17947230
Page seeded by OCA on Thu Feb 21 18:32:40 2008
Categories: Mus musculus | Protein complex | Kanagawa, O. | Kishishita, S. | Kukimoto-Niino, M. | Murayama, K. | Olsen, S K. | Ota, N. | RSGI, RIKEN Structural Genomics/Proteomics Initiative. | Shirouzu, M. | Terada, T. | Yokoyama, S. | BAM | Alternative splicing | Cytokine | Endoplasmic reticulum | Glycoprotein | Golgi apparatus | Membrane | National project on protein structural and functional analyses | Nppsfa | Nucleus | Phosphorylation | Receptor | Riken structural genomics/proteomics initiative | Rsgi | Secreted | Structural genomics | Sushi | Transmembrane