Sandbox Reserved 828
From Proteopedia
| Line 18: | Line 18: | ||
[http://jenalib.fli-leibniz.de/ImgLibPDB/thumbnail/manual/1ab4.pdb1_small.gif Biological Unit] | [http://jenalib.fli-leibniz.de/ImgLibPDB/thumbnail/manual/1ab4.pdb1_small.gif Biological Unit] | ||
| - | [[Image: | + | [[Image:Gyrasegene.jpg]] |
'''The A protein''' breaks and religates DNA as the DNA cleavage core and the CTD lies on it. | '''The A protein''' breaks and religates DNA as the DNA cleavage core and the CTD lies on it. | ||
Revision as of 15:28, 24 December 2013
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
|
Introduction :
Gyrase is the only prokaryote DNA topoisomerase II able to introduce negative supercoils in the DNA in order to remove positive supercoils. It catalyses the hydrolysis of two phosphodiester bonds in a DNA segment (called G segment). Then, thanks to ATP dependant conformation changes it enables the passage of another segment (the T segment) through the break, and then religates the broken segment. Gyrase acts prior to the replication (before the replication fork) or other mecanisms requiring loose DNA. In abscence of ATP, like other topoisomerases II, gyrase only relaxes supercoils.
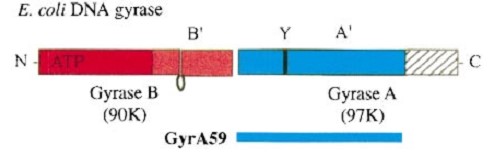
Gyrase is coded by two differents contiguous genes gyrA and GgyrB as it is a 350 kDa A2B2 heterotetramers of two A proteins and two B proteins. The A protein breaks and religates DNA . The B protein has ATPase activity
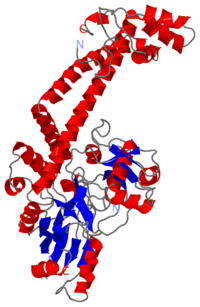
Structure Biological Unit
The A protein breaks and religates DNA as the DNA cleavage core and the CTD lies on it. The gyrA 59kDa N-ter domain is called Breakage and reunion domain It is composed of two domains at the head region : a winged-helix-turn-helix domain or winged helix domain (WHD) where lies the catalytic tyrosines and a tower domain with alpha/beta structure. And a single domain with a helical core at the tail region. Two long helices (a14 anda18) emanate from this core and connect, together with the C-terminal helix (a19), the head and tail fragments. The three connecting helices (a14,a18 anda19) adopt very different conformations, leading to large quaternary movements involving a single hinge-point within the helices and rigid body movements of the head fragments . The tail is structurally conserved although large surface loops emanating from different points give it a different outward appearance It forms a heart-shaped homodimer with two protein interfaces, the DNA- and C-gates. GyrA59 is the minimal fragment of the A-subunit which, when complexed with the B-subunit, has DNA-cleavage activity. The remaining 30–35 kDa comprising the C-terminal domain (CTD) of GyrA shows a domain forming a b-pinwheel with a positively charged amino-acid perimeter. This carboxy-terminal domain of GyrA (cyan) is required for the introduction of DNA supercoils).
The B protein has the ATPase domain and the Toprim fold on it. Two ATPase domains dimerize to form a closed conformation. The Toprim fold is a Rossmann fold that contains three invariant acidic residues that coordinate magnesium ions involved in DNA cleavage and DNA religation
The central core of the protein contains a Toprim fold and a DNA-binding core that contains a winged helix domain (WHD), often referred to as a CAP domain. The catalytic tyrosine lies on this WHD. The DNA-binding core consists of the WHD, which leads to a tower domain. . A coiled-coil region leads to a C-terminal domain that forms the main dimer interface
the Toprim domain is flexible and that this flexibility can allow the Toprim domain to coordinate with the WHD to form a competent cleavage complex
DNA was bent by ~150 degrees through an invariant isoleucine (in topoisomerase II it is I833 and in gyrase it is I172)
The first structure of a C-terminal domain of gyrase was solved by Corbett et al. (Proceedings of the National Academy of Science, 2004, PDB ID = 1SUU)
The structures formed a novel beta barrel, which bends DNA by wrapping the nucleic acid around itself. The bending of DNA by gyrase has been proposed as a key mechanism in the ability of gyrase to introduce negative supercoils into the DNA. This is consistent with footprinting data that shows
that gyrase has a 140-base-pair footprint. Both gyrase and topoisomerase IV CTDs bend DNA, but only gyrase introduces negative supercoils.
Reaction
The cleavage of DNA is achieved by a transesterification reaction between the tyrosines (Tyr122) and the target phosphoryl groups on opposing strands of the DNA backbone, resulting in the tyrosine being covalentlty attached to the 5' end of the cleaved segment with a 4-base overhang. The active site tyrosines (Tyr 122) are on loops at either end of the dimer interface, 30Å apart, and sit at the ends of strongly basic grooves created by the dimer-related monomers.
The gyrase structure reveals a new cluster of conserved residues, juxtaposing Tyr 122 and Arg 121 from one monomer and His 80, Arg 32 and Lys 42 from the other monomer. This cluster may form the active site of the breakage–reunion reaction, with the other conserved positive charges (Arg 46 and Arg 47) anchoring the non-covalently bound 3' end of the cleaved DNA.