Sandbox Reserved 828
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
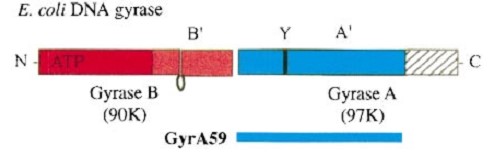
'''The A protein''' breaks and religates DNA as the DNA cleavage core and the CTD lies on it. | '''The A protein''' breaks and religates DNA as the DNA cleavage core and the CTD lies on it. | ||
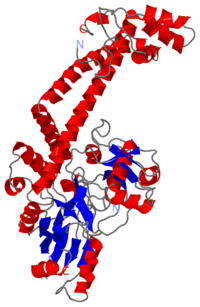
The gyrA 59kDa N-ter domain is called Breakage and reunion domain It is composed of two domains at the head region : a winged-helix-turn-helix domain or winged helix domain (WHD) where lies the catalytic tyrosines and a tower domain with alpha/beta structure. | The gyrA 59kDa N-ter domain is called Breakage and reunion domain It is composed of two domains at the head region : a winged-helix-turn-helix domain or winged helix domain (WHD) where lies the catalytic tyrosines and a tower domain with alpha/beta structure. | ||
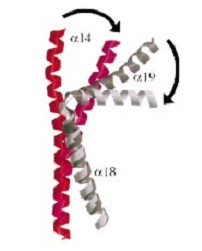
| - | And a single domain with a helical core at the tail region. Two long helices (a14 anda18) emanate from this core and connect, together with the C-terminal helix (a19), the head and tail fragments. | + | And a single domain with a helical core at the tail region. Two long helices (a14 anda18) emanate from this core and connect, together with the C-terminal helix (a19), the head and tail fragments. |
| + | [[Image:gyrA59.jpg]] | ||
| + | |||
| + | |||
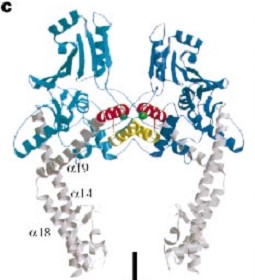
The three connecting helices (a14,a18 anda19) adopt very different conformations, leading to large quaternary movements involving a single hinge-point within the helices and rigid body movements of | The three connecting helices (a14,a18 anda19) adopt very different conformations, leading to large quaternary movements involving a single hinge-point within the helices and rigid body movements of | ||
the head fragments. | the head fragments. | ||
| - | [[Image: | + | [[Image:helixrot.jpg]] [[Image:gyrA592.jpg]] |
The tail is structurally conserved although large surface loops emanating from different points give it a different outward appearance It forms a heart-shaped homodimer with two protein interfaces, the DNA- and C-gates. GyrA59 is the minimal fragment of the A-subunit which, when complexed with the B-subunit, has DNA-cleavage activity. | The tail is structurally conserved although large surface loops emanating from different points give it a different outward appearance It forms a heart-shaped homodimer with two protein interfaces, the DNA- and C-gates. GyrA59 is the minimal fragment of the A-subunit which, when complexed with the B-subunit, has DNA-cleavage activity. | ||
Revision as of 16:15, 24 December 2013
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
| |||||||||||