Sandbox Reserved 828
From Proteopedia
| (26 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| + | |||
| + | ==E.Coli Gyrase== | ||
---- | ---- | ||
| - | + | {{STRUCTURE_1ab4| PDB=1ab4 | CAPTION=A-subunit (monomer), resolution 2.80Å, 1ab4.pdb| SCENE=56/568026/Coloured/1}} | |
[[Image:1ab4.png|left|200px]] | [[Image:1ab4.png|left|200px]] | ||
| - | =='''Introduction | + | ==='''Introduction'''=== |
| - | Gyrase is the '''only prokaryote DNA topoisomerase II able to introduce negative supercoils in the DNA''' in order to remove positive supercoils. It '''catalyses the hydrolysis of two phosphodiester bonds in a DNA segment''' | + | Gyrase is the '''only prokaryote DNA topoisomerase II able to introduce negative supercoils in the DNA''' (see [http://en.wikipedia.org/wiki/DNA_supercoil DNA Supercoil]) in order to remove positive supercoils. It '''catalyses the hydrolysis of two phosphodiester bonds in a DNA segment (DNA cleavage)''' called G segment. Then, thanks to '''ATP dependant conformation changes''', it enables the passage of another segment (the T segment) through the break, and then religates the broken segment. Gyrase acts prior to the replication (before the replication fork) or other mecanisms requiring loose DNA. |
'''In abscence of ATP, like other topoisomerases II, gyrase only relaxes supercoils.''' | '''In abscence of ATP, like other topoisomerases II, gyrase only relaxes supercoils.''' | ||
| - | Gyrase is coded by two differents contiguous genes gyrA and | + | Gyrase is '''coded by two differents contiguous genes gyrA and gyrB''' as it is a 350 kDa '''A<sub>2</sub>B<sub>2</sub> heterotetramers''' of two A proteins and two B proteins. |
| - | The A protein breaks and religates DNA . The B protein has ATPase activity | + | |
| + | The A protein breaks and religates DNA. | ||
| + | The B protein has ATPase activity. | ||
---- | ---- | ||
| - | =='''Structure'''== | + | |
| + | ==='''Structure'''=== | ||
[[Image:Gyrasegene.jpg]] | [[Image:Gyrasegene.jpg]] | ||
| - | '''The A protein''' breaks and religates DNA as the DNA cleavage core and the CTD lies on it. | + | '''The A protein''' breaks and religates DNA as the DNA cleavage core and the CTD lies on it. The smallest fragment capable of catalyzing DNA breakage and reunion is between residues 7 and 523 of A sequence, it is the gyrA59 (59kDa). |
| - | The gyrA | + | The gyrA N-ter domain is called '''breakage and reunion domain'''. It is composed of two domains at the <scene name='56/568026/Head/2'>head</scene> region : |
| + | *a winged-helix-turn-helix domain or <scene name='56/568026/Whd/1'>winged helix domain (WHD</scene>) where lies the catalytic tyrosines: the active-site tyrosines <scene name='56/568026/Tyr122/1'>(Tyr 122)</scene> are on loops at either end of the dimer interface. | ||
| - | + | *a <scene name='56/568026/Tower/1'>tower domain</scene> with alpha/beta structure that participates in DNA bending during cleavage. | |
| - | + | ||
| - | + | ||
| + | The '''‘head’ dimer interface is dominated by an antiparallel side by-side packing of the <scene name='56/568026/Alpha3/2'>alpha 3</scene> (the first helix of the HTH motif) from each monomer''', together with their adjoining loops. At the top of the interface, '''the ‘recognition’ helices <scene name='56/568026/Alpha4/1'>alpha 4</scene> make a head-to-head antiparallel dimer contact.''' | ||
| - | + | ||
| + | There is a '''<scene name='56/568026/Coiledcoil_141819/1'>Coiled-coil</scene> domain''', folowing the tower, with a helical core at the tail region. Two long helices ( | ||
| + | <scene name='56/568026/Helix14/1'>alpha helix 14</scene> and <scene name='56/568026/Helix18/1'>alpha helix 18</scene>) emanate from this core and connect, together with the C-terminal helix (<scene name='56/568026/Helix19/1'>alpha helix 19</scene>), the head and tail fragments. This domain has a small globular domain at it's end and is '''involved in the dimerization creating the <scene name='56/568026/Coiledcoil/1'>C-gate</scene>.''' | ||
| - | The three connecting helices ( | + | The '''three connecting helices (<scene name='56/568026/Helix14/1'>α14</scene>, <scene name='56/568026/Helix18/1'>α18</scene> and <scene name='56/568026/Helix19/1'>α19</scene>)''' adopt very different conformations, leading to '''large quaternary movements''' involving a single hinge-point within the helices and '''rigid body movements of the head fragments.''' |
| - | the head fragments. | + | |
[[Image:helixrot.jpg]] [[Image:gyrA592.jpg]] | [[Image:helixrot.jpg]] [[Image:gyrA592.jpg]] | ||
The tail is structurally conserved although large surface loops emanating from different points give it a different outward appearance. | The tail is structurally conserved although large surface loops emanating from different points give it a different outward appearance. | ||
| - | GyrA forms a heart-shaped homodimer with two protein interfaces, the DNA- and C-gates. GyrA59 is the minimal fragment of the A-subunit which, when complexed with the B-subunit, has DNA-cleavage activity. | + | '''GyrA forms a heart-shaped homodimer with two protein interfaces, the DNA- and C-gates'''. GyrA59 is the minimal fragment of the A-subunit which, when complexed with the B-subunit, has DNA-cleavage activity. |
The remaining 30–35 kDa comprising the C-terminal domain (CTD) of GyrA shows a domain forming a b-pinwheel with a positively charged amino-acid perimeter. This carboxy-terminal domain of GyrA (cyan) is required for the introduction of DNA supercoils), DNA wraps around it. It is linked to the central part of Gyrase A by a flexible 13-15 residues region and stands close to the C-gate and may present an up and down movement in the early stades of the catalytic cycle and may be involved in the helping of G segment and D-gate binding so as the CTD binding of DNA. | The remaining 30–35 kDa comprising the C-terminal domain (CTD) of GyrA shows a domain forming a b-pinwheel with a positively charged amino-acid perimeter. This carboxy-terminal domain of GyrA (cyan) is required for the introduction of DNA supercoils), DNA wraps around it. It is linked to the central part of Gyrase A by a flexible 13-15 residues region and stands close to the C-gate and may present an up and down movement in the early stades of the catalytic cycle and may be involved in the helping of G segment and D-gate binding so as the CTD binding of DNA. | ||
| Line 51: | Line 57: | ||
The Toprim domain is flexible and that this flexibility can allow the Toprim domain to coordinate with | The Toprim domain is flexible and that this flexibility can allow the Toprim domain to coordinate with | ||
the WHD to form a competent cleavage complex. | the WHD to form a competent cleavage complex. | ||
| - | DNA is bent by ~150 degrees through an invariant isoleucine ( | + | DNA is bent by ~150 degrees through an invariant isoleucine (I172) |
| Line 58: | Line 64: | ||
---- | ---- | ||
| - | ''' | + | ==='''Mechanism'''=== |
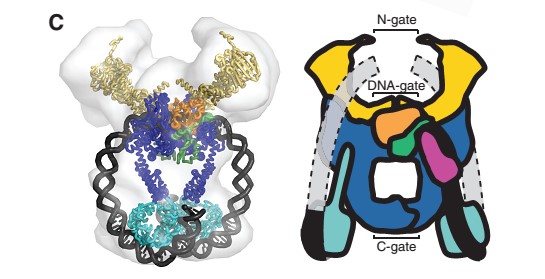
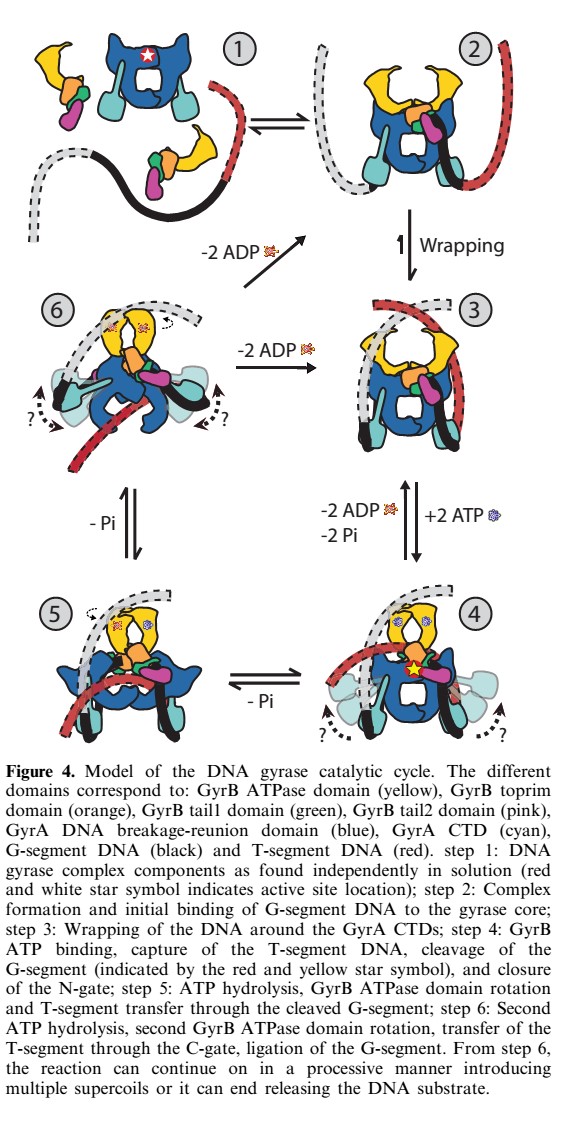
40-bp of duplex DNA, the G-segment, bind to the core of the enzyme and are cleaved by the active site tyrosines, while another DNA duplex, the T-segment, is captured through the ATP-induced dimerization of a protein gate, the N-gate. After passage through the transiently broken G-segment (DNA gate), the T-segment exits the protein through another protein gate, the C-gate. ATP hydrolysis and release reset the conformation of the enzyme and DNA to their initial state, poised for another strand-passage event or release of the DNA. | 40-bp of duplex DNA, the G-segment, bind to the core of the enzyme and are cleaved by the active site tyrosines, while another DNA duplex, the T-segment, is captured through the ATP-induced dimerization of a protein gate, the N-gate. After passage through the transiently broken G-segment (DNA gate), the T-segment exits the protein through another protein gate, the C-gate. ATP hydrolysis and release reset the conformation of the enzyme and DNA to their initial state, poised for another strand-passage event or release of the DNA. | ||
| - | N- and C-gates that open or close to allow T-segment transport through both | + | N- and C-gates that open or close to allow T-segment transport through both the protein and the cleaved G-segment. |
| Line 68: | Line 74: | ||
---- | ---- | ||
| - | =='''Reaction'''== | + | ==='''Reaction'''=== |
| - | The cleavage of DNA is achieved by a '''transesterification reaction between the tyrosines | + | |
| - | The '''active site tyrosines (Tyr 122)''' are on loops at either end of the dimer interface, 30Å apart, and sit at the ends of strongly basic grooves created by the dimer-related monomers. | + | The process of DNA supercoiling by gyrase is the following : |
| + | # Gyrase binds DNA (gyrase binds more strongly to relaxed or linear DNA than to supercoiled one by a factor of about ten) | ||
| + | # Cleavage of the G-segment | ||
| + | # Passage of T-segment of the other strand through the cleavage site (thanks to ATP) | ||
| + | # Reunion of the G segment | ||
| + | # Translocation | ||
| + | |||
| + | The '''A subunit is responsible for the breakage reunion of DNA whereas the B-subunit has the ATPase activity.''' | ||
| + | '''The introduction of negative supercoils requires the energy of ATP-hydrolysis. 2 ATP are hydrolyzed per reaction (2 B-subunits in the gyrase)''' so that the linking number (Lk) changes in step of 2. The '''ATPase activity of the B subunit is partially located in the N-terminal region of protein B whereas the C-terminal is involved in the interaction with the A subunit and DNA.''' | ||
| + | '''The B subunit has a weak ATPase activity in the absence of A and DNA, eventhought the A subunit is able to bind DNA without the B subunit. Both subunits are required for all the reactions of gyrase.''' | ||
| + | The DNA supercoiling reaction '''requires in addition to ATP, a divalent cation such as Mg<sup>2+</sup>''', and is stimulated in the presence of spermidine. | ||
| + | |||
| + | Therefore, the gyrase could also catalyse the following reaction : | ||
| + | * Relaxation of negative/positive supercoils | ||
| + | * Catenation/Decatenation of closed-circular DNA | ||
| + | * DNA cleavage (induced by Quinolone) | ||
| + | * Unknotting DNA strands | ||
| + | |||
| + | Contrary to the introduction of negative supercoils, '''some of these reactions don't necessarily need the energy from ATP-hydrolysis.''' | ||
| + | |||
| + | The cleavage of DNA is achieved by a '''transesterification reaction between the tyrosines <scene name='56/568026/Tyr122/1'>(Tyr 122)</scene> and the target phosphoryl groups on opposing strands of the DNA backbone''', resulting in the tyrosine being covalentlty attached to the 5' end of the cleaved segment with a 4-base overhang. | ||
| + | The '''active site tyrosines <scene name='56/568026/Tyr122/1'>(Tyr 122)</scene>''' are on loops at either end of the dimer interface, 30Å apart, and sit at the ends of strongly basic grooves created by the dimer-related monomers. | ||
The gyrase structure reveals a '''new cluster of conserved residues''', juxtaposing Tyr 122 and Arg 121 from one monomer and His 80, Arg 32 and Lys 42 from the other monomer. '''This cluster may form the active site of the breakage–reunion reaction''', with the other conserved positive charges (Arg 46 and Arg 47) anchoring the non-covalently bound 3' end of the cleaved DNA. | The gyrase structure reveals a '''new cluster of conserved residues''', juxtaposing Tyr 122 and Arg 121 from one monomer and His 80, Arg 32 and Lys 42 from the other monomer. '''This cluster may form the active site of the breakage–reunion reaction''', with the other conserved positive charges (Arg 46 and Arg 47) anchoring the non-covalently bound 3' end of the cleaved DNA. | ||
| - | + | ||
| + | The gyrase could be inhibited by both the [http://en.wikipedia.org/wiki/Quinolone quinolones] and [http://en.wikipedia.org/wiki/Coumarin coumarins]. | ||
| + | |||
| + | '''Coumarins prevent the B-subunit of the gyrase from hydrolyzing the ATP''', so that the gyrase can't come back to its starting state for another round of supercoiling. The mecanism is not yet well known. | ||
| + | |||
| + | '''Quinolones''' inhibit DNA supercoiling by gyrase, it has even the ability to '''induce DNA cleavage''' instead. It appears that '''these drugs will arrest any gyrase rection involving double-strand DNA breakage.''' | ||
| + | However, some mutations offers quinolone resistance, most of them involved a part of the GyrA sequence : between the amino acids 67 and 106. | ||
| + | There are also others antibiotics, like the Cinodine which binds DNA and inhibits its supercoiling by gyrase. | ||
| + | |||
| + | ===See Also=== | ||
| + | *[[Gyrase|Gyrase]] | ||
| + | *[[Topoisomerase|Topoisomerase]] | ||
| + | |||
| + | ===References=== | ||
| + | <ref group="xtra">PMID:009278055</ref><references group="xtra"/> | ||
| + | [[Category: Escherichia coli]] | ||
| + | [[Category: Cabral, J H.M.]] | ||
| + | [[Category: Liddington, R C.]] | ||
| + | [[Category: Maxwell, A.]] | ||
| + | [[Category: Gyrase]] | ||
| + | [[Category: Supercoiling dna]] | ||
| + | [[Category: Topoisomerase]] | ||
| + | [[Category: Topoisomerase ii]] | ||
| + | *Reece, Richard J., Anthony Maxwell, and James C. Wang. "DNA gyrase: structure and function." Critical reviews in biochemistry and molecular biology 26.3-4 (1991): 335-375. | ||
| + | |||
| + | *Nicole M. Baker, Steven Weigand, Sarah Maar-Mathiasand Alfonso Mondrago´n "Solution structures of DNA-bound gyrase" Nucleic Acids Res. 2011 January; 39(2): 755–766. | ||
Current revision
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Contents |
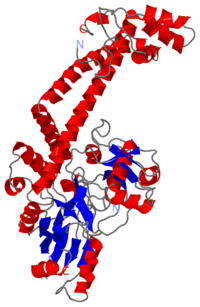
E.Coli Gyrase
| |||||||||
| A-subunit (monomer), resolution 2.80Å, 1ab4.pdb | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Activity: | DNA topoisomerase (ATP-hydrolyzing), with EC number 5.99.1.3 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Introduction
Gyrase is the only prokaryote DNA topoisomerase II able to introduce negative supercoils in the DNA (see DNA Supercoil) in order to remove positive supercoils. It catalyses the hydrolysis of two phosphodiester bonds in a DNA segment (DNA cleavage) called G segment. Then, thanks to ATP dependant conformation changes, it enables the passage of another segment (the T segment) through the break, and then religates the broken segment. Gyrase acts prior to the replication (before the replication fork) or other mecanisms requiring loose DNA. In abscence of ATP, like other topoisomerases II, gyrase only relaxes supercoils.
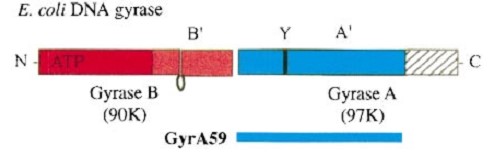
Gyrase is coded by two differents contiguous genes gyrA and gyrB as it is a 350 kDa A2B2 heterotetramers of two A proteins and two B proteins.
The A protein breaks and religates DNA. The B protein has ATPase activity.
Structure
The A protein breaks and religates DNA as the DNA cleavage core and the CTD lies on it. The smallest fragment capable of catalyzing DNA breakage and reunion is between residues 7 and 523 of A sequence, it is the gyrA59 (59kDa). The gyrA N-ter domain is called breakage and reunion domain. It is composed of two domains at the region :
- a winged-helix-turn-helix domain or ) where lies the catalytic tyrosines: the active-site tyrosines are on loops at either end of the dimer interface.
- a with alpha/beta structure that participates in DNA bending during cleavage.
The ‘head’ dimer interface is dominated by an antiparallel side by-side packing of the (the first helix of the HTH motif) from each monomer, together with their adjoining loops. At the top of the interface, the ‘recognition’ helices make a head-to-head antiparallel dimer contact.
There is a domain, folowing the tower, with a helical core at the tail region. Two long helices (
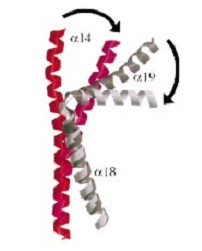
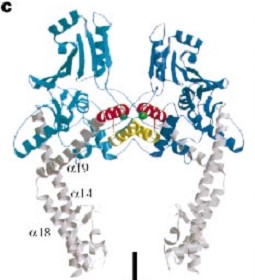
and ) emanate from this core and connect, together with the C-terminal helix (), the head and tail fragments. This domain has a small globular domain at it's end and is involved in the dimerization creating the .
The three connecting helices (, and ) adopt very different conformations, leading to large quaternary movements involving a single hinge-point within the helices and rigid body movements of the head fragments.
The tail is structurally conserved although large surface loops emanating from different points give it a different outward appearance. GyrA forms a heart-shaped homodimer with two protein interfaces, the DNA- and C-gates. GyrA59 is the minimal fragment of the A-subunit which, when complexed with the B-subunit, has DNA-cleavage activity. The remaining 30–35 kDa comprising the C-terminal domain (CTD) of GyrA shows a domain forming a b-pinwheel with a positively charged amino-acid perimeter. This carboxy-terminal domain of GyrA (cyan) is required for the introduction of DNA supercoils), DNA wraps around it. It is linked to the central part of Gyrase A by a flexible 13-15 residues region and stands close to the C-gate and may present an up and down movement in the early stades of the catalytic cycle and may be involved in the helping of G segment and D-gate binding so as the CTD binding of DNA.
The B protein has the GHKL ATPase domain, a transducer domain and the Toprim fold on it. Two ATPase domains from two monomers dimerize to form a closed conformation, the N-gate. The Toprim fold is a Rossmann fold (beta-alpha-beta-alpha-beta) that contains three invariant acidic residues that coordinate magnesium ions involved in DNA cleavage and DNA religation. This torpim fold interacts with the Winged helix domain of gyrA to create the D-gate.
The central core of the protein is made out of the association of GyrA and Gyr B with the Toprim fold, the DNA-binding domain (WHD)and tower domain, all three creating the D-gate.
These domains are composed of numerous positively charged residues to interact with DNA.
The Toprim domain is flexible and that this flexibility can allow the Toprim domain to coordinate with
the WHD to form a competent cleavage complex.
DNA is bent by ~150 degrees through an invariant isoleucine (I172)
Mechanism
40-bp of duplex DNA, the G-segment, bind to the core of the enzyme and are cleaved by the active site tyrosines, while another DNA duplex, the T-segment, is captured through the ATP-induced dimerization of a protein gate, the N-gate. After passage through the transiently broken G-segment (DNA gate), the T-segment exits the protein through another protein gate, the C-gate. ATP hydrolysis and release reset the conformation of the enzyme and DNA to their initial state, poised for another strand-passage event or release of the DNA. N- and C-gates that open or close to allow T-segment transport through both the protein and the cleaved G-segment.
Reaction
The process of DNA supercoiling by gyrase is the following :
- Gyrase binds DNA (gyrase binds more strongly to relaxed or linear DNA than to supercoiled one by a factor of about ten)
- Cleavage of the G-segment
- Passage of T-segment of the other strand through the cleavage site (thanks to ATP)
- Reunion of the G segment
- Translocation
The A subunit is responsible for the breakage reunion of DNA whereas the B-subunit has the ATPase activity. The introduction of negative supercoils requires the energy of ATP-hydrolysis. 2 ATP are hydrolyzed per reaction (2 B-subunits in the gyrase) so that the linking number (Lk) changes in step of 2. The ATPase activity of the B subunit is partially located in the N-terminal region of protein B whereas the C-terminal is involved in the interaction with the A subunit and DNA. The B subunit has a weak ATPase activity in the absence of A and DNA, eventhought the A subunit is able to bind DNA without the B subunit. Both subunits are required for all the reactions of gyrase. The DNA supercoiling reaction requires in addition to ATP, a divalent cation such as Mg2+, and is stimulated in the presence of spermidine.
Therefore, the gyrase could also catalyse the following reaction :
- Relaxation of negative/positive supercoils
- Catenation/Decatenation of closed-circular DNA
- DNA cleavage (induced by Quinolone)
- Unknotting DNA strands
Contrary to the introduction of negative supercoils, some of these reactions don't necessarily need the energy from ATP-hydrolysis.
The cleavage of DNA is achieved by a transesterification reaction between the tyrosines and the target phosphoryl groups on opposing strands of the DNA backbone, resulting in the tyrosine being covalentlty attached to the 5' end of the cleaved segment with a 4-base overhang. The active site tyrosines are on loops at either end of the dimer interface, 30Å apart, and sit at the ends of strongly basic grooves created by the dimer-related monomers.
The gyrase structure reveals a new cluster of conserved residues, juxtaposing Tyr 122 and Arg 121 from one monomer and His 80, Arg 32 and Lys 42 from the other monomer. This cluster may form the active site of the breakage–reunion reaction, with the other conserved positive charges (Arg 46 and Arg 47) anchoring the non-covalently bound 3' end of the cleaved DNA.
The gyrase could be inhibited by both the quinolones and coumarins.
Coumarins prevent the B-subunit of the gyrase from hydrolyzing the ATP, so that the gyrase can't come back to its starting state for another round of supercoiling. The mecanism is not yet well known.
Quinolones inhibit DNA supercoiling by gyrase, it has even the ability to induce DNA cleavage instead. It appears that these drugs will arrest any gyrase rection involving double-strand DNA breakage. However, some mutations offers quinolone resistance, most of them involved a part of the GyrA sequence : between the amino acids 67 and 106. There are also others antibiotics, like the Cinodine which binds DNA and inhibits its supercoiling by gyrase.
See Also
References
- Morais Cabral JH, Jackson AP, Smith CV, Shikotra N, Maxwell A, Liddington RC. Crystal structure of the breakage-reunion domain of DNA gyrase. Nature. 1997 Aug 28;388(6645):903-6. PMID:9278055 doi:10.1038/42294
- Reece, Richard J., Anthony Maxwell, and James C. Wang. "DNA gyrase: structure and function." Critical reviews in biochemistry and molecular biology 26.3-4 (1991): 335-375.
- Nicole M. Baker, Steven Weigand, Sarah Maar-Mathiasand Alfonso Mondrago´n "Solution structures of DNA-bound gyrase" Nucleic Acids Res. 2011 January; 39(2): 755–766.