User:Cody Couperus/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 22: | Line 22: | ||
==The Thrombin Life Cycle== | ==The Thrombin Life Cycle== | ||
| - | Upon tissue damage [http://en.wikipedia.org/wiki/Tissue_factor tissue factor (TF)] is released by subendothelial cells. This interacts with circulating [[Factor_VII| FVIIa]], a zymogen-like serine protease, significantly increasing its activity. The FVIIa-TF complex activates FVII, FIX, and FX. The now active FXa cleaves prothrombin bound to membranes through its [http://en.wikipedia.org/wiki/Gla_domain Gla domain], activating it to thrombin. | + | Upon tissue damage [http://en.wikipedia.org/wiki/Tissue_factor tissue factor (TF)] is released by subendothelial cells. This interacts with circulating [[Factor_VII| FVIIa]], a zymogen-like serine protease, significantly increasing its activity. '''The FVIIa-TF complex activates FVII, FIX, and FX'''. The now active '''FXa cleaves prothrombin''' bound to membranes through its [http://en.wikipedia.org/wiki/Gla_domain Gla domain], activating it to thrombin. |
| - | Thrombin interacts with platelet membrane protein [http://www.ncbi.nlm.nih.gov/pubmed/14720584 GpIbα] and subsequently | + | Thrombin interacts with platelet membrane protein [http://www.ncbi.nlm.nih.gov/pubmed/14720584 GpIbα] and subsequently '''cleaves protease activated receptor-1 (PAR1)''' causing a signaling cascade which leads to platelet [http://en.wikipedia.org/wiki/Platelet_alpha-granule α-granule] release and membrane flipping exposing the negatively charged [http://en.wikipedia.org/wiki/Phosphatidylserine phosphatidylserine]. The platelet alpha granules contain the physiologically relevant pool of FVa. <ref>Camire, R. M. (2010). Platelet factor V to the rescue. Blood, 115(4), 753-754. [http://bloodjournal.hematologylibrary.org/content/115/4/753.full DOI: 10.1182/blood-2009-11-252619]</ref> |
| - | Thrombin also causes activation of FX, through [http://en.wikipedia.org/wiki/ | + | Thrombin also causes activation of FX, through [http://en.wikipedia.org/wiki/Factor_XI FXI] cleavage, and FVIII which form the Xase complex to activate FX. '''FVa and FXa form the [http://en.wikipedia.org/wiki/Prothrombinase prothrombinase] complex''' in the presence of calcium and phospholipid. It causes rapid activation of prothrombin to thrombin. This increase in thrombin allows sufficient fibrinogen to be cleaved to fibrin which is able to polymerize to form a stable [http://en.wikipedia.org/wiki/Thrombus blood clot]. |
| - | Further supporting coagulation, thrombin activates FXIII, a [http://en.wikipedia.org/wiki/Transglutaminase transglutaminase] that crosslinks fibrin at lysine residues. | + | Further supporting coagulation, '''thrombin activates FXIII''', a [http://en.wikipedia.org/wiki/Transglutaminase transglutaminase] that crosslinks fibrin at lysine residues. |
| - | The structure of thrombin facilitates its inactivation. Once the endothelial lining is reached thrombin binds [http://en.wikipedia.org/wiki/Heparin heparin] [http://en.wikipedia.org/wiki/Glycosaminoglycan glycosaminoglycans]. | + | The structure of thrombin facilitates its inactivation. Once the endothelial lining is reached '''thrombin binds [http://en.wikipedia.org/wiki/Heparin heparin] [http://en.wikipedia.org/wiki/Glycosaminoglycan glycosaminoglycans]'''. Th'''is facilitates its inactivation by serpin inhibitors antithrombin and heparin cofactor II. In addition, thrombin will interact with [http://en.wikipedia.org/wiki/Thrombomodulin thrombomodulin]''' which significantly increases its catalytic efficiency activating protein C. Activated protein C inactivates FVIIa and FVa thus down regulating thrombin generation. |
| Line 38: | Line 38: | ||
[[Image:Prothrombin activation scheme_3.png|300px|right|thumb| Activation scheme of in vivo activation of prothrombin by FXa in the absence of FVa.]] | [[Image:Prothrombin activation scheme_3.png|300px|right|thumb| Activation scheme of in vivo activation of prothrombin by FXa in the absence of FVa.]] | ||
[[Image:Prothrombin activation scheme_nofva3.png|300px|right|thumb| Activation scheme of in vivo activation of prothrombin by the prothrombinase complex in presence of calcium and a phospholipid bilayer]] | [[Image:Prothrombin activation scheme_nofva3.png|300px|right|thumb| Activation scheme of in vivo activation of prothrombin by the prothrombinase complex in presence of calcium and a phospholipid bilayer]] | ||
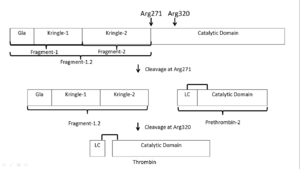
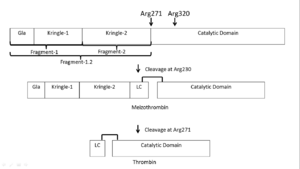
| - | Prothrombin is the zymogen form of thrombin. From N-terminal to C-terminal it consists of a Gla domain, two kringle domains, and a catalytic domain. | + | Prothrombin is the zymogen form of thrombin. From N-terminal to C-terminal it consists of a Gla domain, two kringle domains, and a catalytic domain. The Gla domain is formed by vitamin K dependent carboxylation of glutamate residues.<ref>PMID: 18374193</ref> |
| - | Prothrombin is activated by prothrombinase which consists of FXa, FVa, calcium, and a phospholipid surface. In vivo the first cleavage occurs at the R320-I321 bond, corresponding to residues 15-16 in thrombin which is the N-terminus of the B chain, producing meizothrombin.<ref name='seven'>PMID: 22944689</ref> Subsequent cleavage at R271-T272 yields thrombin.<ref name='seven'/> The initial cleavage can also occur at R271 resulting in prethrombin-2 which will then be cleaved at R320 to produce thrombin.<ref>PMID: 1995649</ref> | + | '''Prothrombin is activated by prothrombinase''' which consists of FXa, FVa, calcium, and a phospholipid surface. In vivo the first cleavage occurs at the R320-I321 bond, corresponding to residues 15-16 in thrombin which is the N-terminus of the B chain, producing meizothrombin.<ref name='seven'>PMID: 22944689</ref> Subsequent cleavage at R271-T272 yields thrombin.<ref name='seven'/> The initial cleavage can also occur at R271 resulting in prethrombin-2 which will then be cleaved at R320 to produce thrombin.<ref>PMID: 1995649</ref> |
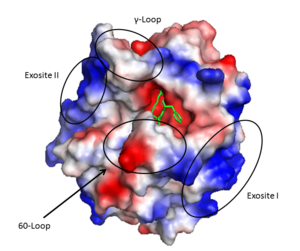
After cleavage by prothrombinase the new B chain N-terminus (Ile16) folds into the core protease domain and forms a salt bridge with Asp194.<ref name='seven'/> This leads to stabilization of regions of the 180s-loop, Na+ binding loop, and γ-loop (zymogen activation domains). These changes provide the correct conformation for the S1 pocket and oxyanion hole for catalysis.<ref name='seven'/><ref>PMID: 15890651</ref> | After cleavage by prothrombinase the new B chain N-terminus (Ile16) folds into the core protease domain and forms a salt bridge with Asp194.<ref name='seven'/> This leads to stabilization of regions of the 180s-loop, Na+ binding loop, and γ-loop (zymogen activation domains). These changes provide the correct conformation for the S1 pocket and oxyanion hole for catalysis.<ref name='seven'/><ref>PMID: 15890651</ref> | ||
Revision as of 12:49, 30 April 2014
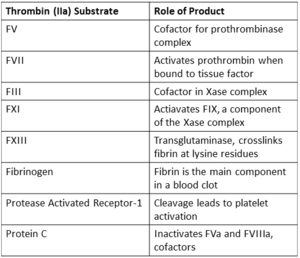
| |||||||||||
References
- ↑ Fenton JW 2nd. Thrombin specificity. Ann N Y Acad Sci. 1981;370:468-95. PMID:7023326
- ↑ 2.0 2.1 Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000 Sep 14;407(6801):258-64. PMID:11001069 doi:http://dx.doi.org/10.1038/35025229
- ↑ Crawley JT, Lam JK, Rance JB, Mollica LR, O'Donnell JS, Lane DA. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood. 2005 Feb 1;105(3):1085-93. Epub 2004 Sep 23. PMID:15388580 doi:http://dx.doi.org/10.1182/blood-2004-03-1101
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Lane DA, Philippou H, Huntington JA. Directing thrombin. Blood. 2005 Oct 15;106(8):2605-12. Epub 2005 Jun 30. PMID:15994286 doi:http://dx.doi.org/10.1182/blood-2005-04-1710
- ↑ Takagi T, Doolittle RF. Amino acid sequence studies on factor XIII and the peptide released during its activation by thrombin. Biochemistry. 1974 Feb 12;13(4):750-6. PMID:4811064
- ↑ Miljic P, Heylen E, Willemse J, Djordjevic V, Radojkovic D, Colovic M, Elezovic I, Hendriks D. Thrombin activatable fibrinolysis inhibitor (TAFI): a molecular link between coagulation and fibrinolysis. Srp Arh Celok Lek. 2010 Jan;138 Suppl 1:74-8. PMID:20229688
- ↑ 7.0 7.1 7.2 7.3 Huntington JA. Natural inhibitors of thrombin. Thromb Haemost. 2014 Apr 1;111(4):583-9. doi: 10.1160/TH13-10-0811. Epub 2014 Jan, 30. PMID:24477356 doi:http://dx.doi.org/10.1160/TH13-10-0811
- ↑ 8.0 8.1 8.2 8.3 8.4 Huntington JA. Thrombin inhibition by the serpins. J Thromb Haemost. 2013 Jun;11 Suppl 1:254-64. doi: 10.1111/jth.12252. PMID:23809129 doi:http://dx.doi.org/10.1111/jth.12252
- ↑ Esmon CT. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348-52. PMID:3029867
- ↑ Kalafatis M, Rand MD, Mann KG. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J Biol Chem. 1994 Dec 16;269(50):31869-80. PMID:7989361
- ↑ 11.0 11.1 Lu D, Kalafatis M, Mann KG, Long GL. Comparison of activated protein C/protein S-mediated inactivation of human factor VIII and factor V. Blood. 1996 Jun 1;87(11):4708-17. PMID:8639840
- ↑ Duga S, Asselta R, Tenchini ML. Coagulation factor V. Int J Biochem Cell Biol. 2004 Aug;36(8):1393-9. PMID:15147718 doi:http://dx.doi.org/10.1016/j.biocel.2003.08.002
- ↑ Saenko EL, Shima M, Sarafanov AG. Role of activation of the coagulation factor VIII in interaction with vWf, phospholipid, and functioning within the factor Xase complex. Trends Cardiovasc Med. 1999 Oct;9(7):185-92. PMID:10881749
- ↑ Camire, R. M. (2010). Platelet factor V to the rescue. Blood, 115(4), 753-754. DOI: 10.1182/blood-2009-11-252619
- ↑ Berkner KL. Vitamin K-dependent carboxylation. Vitam Horm. 2008;78:131-56. doi: 10.1016/S0083-6729(07)00007-6. PMID:18374193 doi:http://dx.doi.org/10.1016/S0083-6729(07)00007-6
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 Lechtenberg BC, Freund SM, Huntington JA. An ensemble view of thrombin allostery. Biol Chem. 2012 Sep;393(9):889-98. doi: 10.1515/hsz-2012-0178. PMID:22944689 doi:http://dx.doi.org/10.1515/hsz-2012-0178
- ↑ Tijburg PN, van Heerde WL, Leenhouts HM, Hessing M, Bouma BN, de Groot PG. Formation of meizothrombin as intermediate in factor Xa-catalyzed prothrombin activation on endothelial cells. The influence of thrombin on the reaction mechanism. J Biol Chem. 1991 Feb 25;266(6):4017-22. PMID:1995649
- ↑ Bobofchak KM, Pineda AO, Mathews FS, Di Cera E. Energetic and structural consequences of perturbing Gly-193 in the oxyanion hole of serine proteases. J Biol Chem. 2005 Jul 8;280(27):25644-50. Epub 2005 May 12. PMID:15890651 doi:http://dx.doi.org/10.1074/jbc.M503499200
- ↑ 19.0 19.1 19.2 19.3 Bode W, Mayr I, Baumann U, Huber R, Stone SR, Hofsteenge J. The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989 Nov;8(11):3467-75. PMID:2583108
- ↑ Page MJ, Di Cera E. Evolution of peptidase diversity. J Biol Chem. 2008 Oct 31;283(44):30010-4. doi: 10.1074/jbc.M804650200. Epub 2008 , Sep 3. PMID:18768474 doi:http://dx.doi.org/10.1074/jbc.M804650200
- ↑ Schechter I, Berger A. On the size of the active site in proteases. I. Papain. 1967. Biochem Biophys Res Commun. 2012 Aug 31;425(3):497-502. doi:, 10.1016/j.bbrc.2012.08.015. PMID:22925665 doi:http://dx.doi.org/10.1016/j.bbrc.2012.08.015
- ↑ Huntington JA. Molecular recognition mechanisms of thrombin. J Thromb Haemost. 2005 Aug;3(8):1861-72. PMID:16102053 doi:http://dx.doi.org/10.1111/j.1538-7836.2005.01363.x
- ↑ Zhang E, Tulinsky A. The molecular environment of the Na+ binding site of thrombin. Biophys Chem. 1997 Jan 31;63(2-3):185-200. PMID:9108691
- ↑ Li W, Johnson DJ, Esmon CT, Huntington JA. Structure of the antithrombin-thrombin-heparin ternary complex reveals the antithrombotic mechanism of heparin. Nat Struct Mol Biol. 2004 Sep;11(9):857-62. Epub 2004 Aug 15. PMID:15311269 doi:10.1038/nsmb811
- ↑ Spronk HM, Borissoff JI, ten Cate H. New insights into modulation of thrombin formation. Curr Atheroscler Rep. 2013 Nov;15(11):363. doi: 10.1007/s11883-013-0363-3. PMID:24026641 doi:http://dx.doi.org/10.1007/s11883-013-0363-3