User:Cody Couperus/Sandbox 1
From Proteopedia
< User:Cody Couperus(Difference between revisions)
| (11 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
<StructureSection load='1PPB' size='350' side='right' caption='Thrombin (1PPB) viewed in cartoon representation colored by secondary structure. Active site residues Ser195, Asp102, and His57 are viewed in ball and stick form.' scene='58/583418/Thombin_main_secondary/2'> | <StructureSection load='1PPB' size='350' side='right' caption='Thrombin (1PPB) viewed in cartoon representation colored by secondary structure. Active site residues Ser195, Asp102, and His57 are viewed in ball and stick form.' scene='58/583418/Thombin_main_secondary/2'> | ||
==Introduction== | ==Introduction== | ||
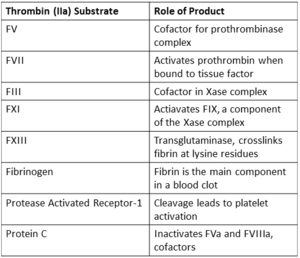
| - | <scene name='58/583418/Thombin_main_secondary/2'>Thrombin</scene> is the serine protease that catalyzes the penultimate step in blood coagulation. It is activated from its [http://en.wikipedia.org/wiki/Zymogen zymogen], prothrombin, at the site of tissue injury by [[Factor_Xa | FXa]] and its cofactor [http://en.wikipedia.org/wiki/Factor_V FVa] in the presence of phospholipid membrane and calcium. Thrombin is then able to catalyze the cleavage of [[Fibrinogen | fibrinogen]] to insoluable fibrin which spontaneously polymerizes to form a stable clot.<ref name="zero">PMID: 7023326</ref><ref name="one">PMID: 11001069</ref> Thrombin also acts as a procoagulant by: | + | <scene name='58/583418/Thombin_main_secondary/2'>Thrombin</scene> is the serine protease that catalyzes the penultimate step in blood coagulation. It is activated from its [http://en.wikipedia.org/wiki/Zymogen zymogen], prothrombin, at the site of tissue injury by [[Factor_Xa | Factor Xa (FXa)]] and its cofactor [http://en.wikipedia.org/wiki/Factor_V FVa] in the presence of phospholipid membrane and calcium. Thrombin is then able to catalyze the cleavage of [[Fibrinogen | fibrinogen]] to insoluable fibrin which spontaneously polymerizes to form a stable clot.<ref name="zero">PMID: 7023326</ref><ref name="one">PMID: 11001069</ref> Thrombin also acts as a procoagulant by: |
* Activating platelets through their [http://en.wikipedia.org/wiki/Protease-activated_receptor protease activated receptors (PARs)]<ref name="one"/> | * Activating platelets through their [http://en.wikipedia.org/wiki/Protease-activated_receptor protease activated receptors (PARs)]<ref name="one"/> | ||
* Preventing [http://en.wikipedia.org/wiki/Von_Willebrand_factor Von Willebrand factor (VWF)] processing by cleaving [http://en.wikipedia.org/wiki/ADAMTS13 ADAMTS13] <ref name="two">PMID: 15388580</ref><ref name="three">PMID: 15994286</ref> | * Preventing [http://en.wikipedia.org/wiki/Von_Willebrand_factor Von Willebrand factor (VWF)] processing by cleaving [http://en.wikipedia.org/wiki/ADAMTS13 ADAMTS13] <ref name="two">PMID: 15388580</ref><ref name="three">PMID: 15994286</ref> | ||
| - | * Enhancing its own production through [[ | + | * Enhancing its own production through [[Factor_XI | FXI]] activation <ref name="three"/> |
* Activating the fibrin crosslinking transglutaminase [[Factor_XIII | FXIII]] <ref>PMID: 4811064</ref> | * Activating the fibrin crosslinking transglutaminase [[Factor_XIII | FXIII]] <ref>PMID: 4811064</ref> | ||
* Activation of [http://www.ncbi.nlm.nih.gov/pubmed/17008302 thrombin activatable fibrinolysis inhibitor (TAFI)] <ref>PMID: 20229688</ref> | * Activation of [http://www.ncbi.nlm.nih.gov/pubmed/17008302 thrombin activatable fibrinolysis inhibitor (TAFI)] <ref>PMID: 20229688</ref> | ||
| Line 15: | Line 15: | ||
* [http://www.sciencedirect.com/science/article/pii/0968000484900744 Protease nexin 1]<ref name="three"/><ref name='four'/><ref name='five'/> | * [http://www.sciencedirect.com/science/article/pii/0968000484900744 Protease nexin 1]<ref name="three"/><ref name='four'/><ref name='five'/> | ||
| - | Generation of thrombin is decreased when thrombin reaches endothelial lining and interacts with [[1fge |thrombomodulin]] which significantly increases activity of thrombin in activating [http://en.wikipedia.org/wiki/Protein_C protein C (APC)].<ref>PMID: 3029867</ref> This enzyme will go on to inactivate FVa<ref>PMID: 7989361</ref><ref name='six'>PMID: 8639840</ref> and [[Factor_VIII | FVIIIa]]<ref name='six'/> , cofactors for activation of prothrombin<ref>PMID: 15147718</ref> and [[Factor_Xa | FXa]]<ref>PMID: 10881749</ref>, respectively. | + | Generation of thrombin is decreased when thrombin reaches endothelial lining and interacts with [[1fge |thrombomodulin]] which significantly increases activity of thrombin in activating [http://en.wikipedia.org/wiki/Protein_C protein C (PC -> APC)].<ref>PMID: 3029867</ref> This enzyme will go on to inactivate FVa<ref>PMID: 7989361</ref><ref name='six'>PMID: 8639840</ref> and [[Factor_VIII | FVIIIa]]<ref name='six'/> , cofactors for activation of prothrombin<ref>PMID: 15147718</ref> and [[Factor_Xa | FXa]]<ref>PMID: 10881749</ref>, respectively. |
By balancing substrate specificity, activity, and inhibition thrombin plays a central role in the blood coagulation cascade. <ref name="three"/> | By balancing substrate specificity, activity, and inhibition thrombin plays a central role in the blood coagulation cascade. <ref name="three"/> | ||
| Line 22: | Line 22: | ||
==The Thrombin Life Cycle== | ==The Thrombin Life Cycle== | ||
| - | Upon tissue damage [http://en.wikipedia.org/wiki/Tissue_factor tissue factor (TF)] is released by subendothelial cells. This interacts with circulating [[Factor_VII| FVIIa]], a zymogen-like serine protease, significantly increasing its activity. '''The FVIIa-TF complex activates FVII, FIX, and FX'''. The now active '''FXa cleaves prothrombin''' bound to membranes through its [http://en.wikipedia.org/wiki/Gla_domain Gla domain], activating it to thrombin. | + | Upon tissue damage [http://en.wikipedia.org/wiki/Tissue_factor tissue factor (TF)] is released by subendothelial cells. This interacts with circulating [[Factor_VII| FVIIa]], a zymogen-like serine protease, significantly increasing its activity. '''The FVIIa-TF complex (extrinsic Xase) activates FVII, FIX, and FX'''. The now active '''FXa cleaves prothrombin''' bound to membranes through its [http://en.wikipedia.org/wiki/Gla_domain gamma-carboxyglutamyl (Gla) domain], activating it to thrombin. |
Thrombin interacts with platelet membrane protein [http://www.ncbi.nlm.nih.gov/pubmed/14720584 GpIbα] and subsequently '''cleaves protease activated receptor-1 (PAR1)''' causing a signaling cascade which leads to platelet [http://en.wikipedia.org/wiki/Platelet_alpha-granule α-granule] release and membrane flipping exposing the negatively charged [http://en.wikipedia.org/wiki/Phosphatidylserine phosphatidylserine]. The platelet alpha granules contain the physiologically relevant pool of FVa. <ref>Camire, R. M. (2010). Platelet factor V to the rescue. Blood, 115(4), 753-754. [http://bloodjournal.hematologylibrary.org/content/115/4/753.full DOI: 10.1182/blood-2009-11-252619]</ref> | Thrombin interacts with platelet membrane protein [http://www.ncbi.nlm.nih.gov/pubmed/14720584 GpIbα] and subsequently '''cleaves protease activated receptor-1 (PAR1)''' causing a signaling cascade which leads to platelet [http://en.wikipedia.org/wiki/Platelet_alpha-granule α-granule] release and membrane flipping exposing the negatively charged [http://en.wikipedia.org/wiki/Phosphatidylserine phosphatidylserine]. The platelet alpha granules contain the physiologically relevant pool of FVa. <ref>Camire, R. M. (2010). Platelet factor V to the rescue. Blood, 115(4), 753-754. [http://bloodjournal.hematologylibrary.org/content/115/4/753.full DOI: 10.1182/blood-2009-11-252619]</ref> | ||
| - | Thrombin also causes activation of | + | Thrombin also causes activation of FIX, through [http://en.wikipedia.org/wiki/Factor_XI FXI] cleavage, and FVIII which form the Xase complex to activate FX. '''FVa and FXa form the [http://en.wikipedia.org/wiki/Prothrombinase prothrombinase] complex''' in the presence of calcium and phospholipid. It causes rapid activation of prothrombin to thrombin. This increase in thrombin allows sufficient fibrinogen to be cleaved to fibrin which is able to polymerize to form a stable [http://en.wikipedia.org/wiki/Thrombus blood clot]. |
Further supporting coagulation, '''thrombin activates FXIII''', a [http://en.wikipedia.org/wiki/Transglutaminase transglutaminase] that crosslinks fibrin at lysine residues. | Further supporting coagulation, '''thrombin activates FXIII''', a [http://en.wikipedia.org/wiki/Transglutaminase transglutaminase] that crosslinks fibrin at lysine residues. | ||
| - | The structure of thrombin facilitates its inactivation. Once the endothelial lining is reached '''thrombin binds [http://en.wikipedia.org/wiki/Heparin heparin] | + | The structure of thrombin facilitates its inactivation. Once the endothelial lining is reached '''thrombin binds [http://en.wikipedia.org/wiki/Heparin heparin] or related [http://en.wikipedia.org/wiki/Glycosaminoglycan glycosaminoglycans]'''. This facilitates its inactivation by serpin inhibitors antithrombin and heparin cofactor II. In addition, '''thrombin will interact with [http://en.wikipedia.org/wiki/Thrombomodulin thrombomodulin]''' which significantly increases its catalytic efficiency activating protein C. '''Activated protein C inactivates FVIIa and FVa''' thus down regulating thrombin generation. |
| Line 40: | Line 40: | ||
Prothrombin is the zymogen form of thrombin. From N-terminal to C-terminal it consists of a Gla domain, two kringle domains, and a catalytic domain. The Gla domain is formed by vitamin K dependent carboxylation of glutamate residues.<ref>PMID: 18374193</ref> | Prothrombin is the zymogen form of thrombin. From N-terminal to C-terminal it consists of a Gla domain, two kringle domains, and a catalytic domain. The Gla domain is formed by vitamin K dependent carboxylation of glutamate residues.<ref>PMID: 18374193</ref> | ||
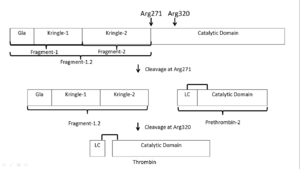
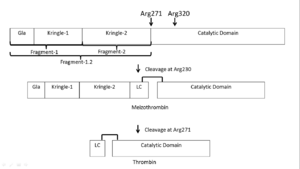
| - | '''Prothrombin is activated by prothrombinase''' which consists of FXa, FVa, calcium, and a phospholipid surface. In vivo the first cleavage occurs at the R320-I321 bond, corresponding to residues 15-16 in | + | '''Prothrombin is activated by prothrombinase''' which consists of FXa, FVa, calcium, and a phospholipid surface. In vivo the first cleavage occurs at the R320-I321 bond, corresponding to residues 15-16 in chymotrypsin which is the N-terminus of the B chain, producing meizothrombin.<ref name='seven'>PMID: 22944689</ref> Subsequent cleavage at R271-T272 yields thrombin.<ref name='seven'/> The initial cleavage can also occur at R271 resulting in prethrombin-2 which will then be cleaved at R320 to produce thrombin.<ref>PMID: 1995649</ref> |
After cleavage by prothrombinase the new B chain '''N-terminus (Ile16) folds into the core protease domain''' and forms a salt bridge with Asp194.<ref name='seven'/> This leads to stabilization of regions of the 180s-loop, Na+ binding loop, and γ-loop (zymogen activation domains). These changes provide the '''correct conformation for the S1 pocket and oxyanion hole for catalysis'''.<ref name='seven'/><ref>PMID: 15890651</ref> | After cleavage by prothrombinase the new B chain '''N-terminus (Ile16) folds into the core protease domain''' and forms a salt bridge with Asp194.<ref name='seven'/> This leads to stabilization of regions of the 180s-loop, Na+ binding loop, and γ-loop (zymogen activation domains). These changes provide the '''correct conformation for the S1 pocket and oxyanion hole for catalysis'''.<ref name='seven'/><ref>PMID: 15890651</ref> | ||
| Line 60: | Line 60: | ||
The serine protease <scene name='58/583418/Catalytic_triad/1'>catalytic triad</scene> ([http://en.wikipedia.org/wiki/Catalytic_triad wiki]) residues, based on chymotrypsin numbering, are Ser195, His57, and Asp102. As is common with serine proteases, an <scene name='58/583418/Oxyanion_hole/2'>oxyanion hole</scene> hole is formed by backbone amides of Ser195 and Gly193.<ref name='seven'/> This has the functional role of stabilizing the oxyanion intermediate involved in the serine protease mechanism by hydrogen bonding to the oxygen of the P1 residue (standard substrate-protease nomeclature <ref>PMID: 22925665</ref>. In addition, since thrombin cleaves after Arg/Lys the <scene name='58/583418/S1/3'>S1 specificity site</scene>, formed by the 180s- and 220s- loops, has Asp189 at the base to form a salt bridge with the incoming substrate. Furthermore, the S4 binding pocket accommodates hydrophobic substrate residues. | The serine protease <scene name='58/583418/Catalytic_triad/1'>catalytic triad</scene> ([http://en.wikipedia.org/wiki/Catalytic_triad wiki]) residues, based on chymotrypsin numbering, are Ser195, His57, and Asp102. As is common with serine proteases, an <scene name='58/583418/Oxyanion_hole/2'>oxyanion hole</scene> hole is formed by backbone amides of Ser195 and Gly193.<ref name='seven'/> This has the functional role of stabilizing the oxyanion intermediate involved in the serine protease mechanism by hydrogen bonding to the oxygen of the P1 residue (standard substrate-protease nomeclature <ref>PMID: 22925665</ref>. In addition, since thrombin cleaves after Arg/Lys the <scene name='58/583418/S1/3'>S1 specificity site</scene>, formed by the 180s- and 220s- loops, has Asp189 at the base to form a salt bridge with the incoming substrate. Furthermore, the S4 binding pocket accommodates hydrophobic substrate residues. | ||
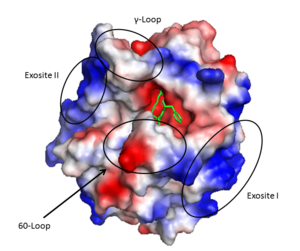
| - | The <scene name='58/583418/Loops/3'>active site</scene> cleft rims are formed by the hydrophobic and rigid <scene name='58/583418/Loops/3'>60- | + | The <scene name='58/583418/Loops/3'>active site</scene> cleft rims are formed by the hydrophobic and rigid <scene name='58/583418/Loops/3'>60-loop</scene> (residues L60, Y60a, P60b, P60c, W60d, D60e, K60f, N60g, F60h, T60i, and N60g) and the <scene name='58/583418/Loops/3'>γ-loop</scene> (residues T147, W147a, T147b, A147c, N147d, and V147f) while the base is mostly hydrophilic negatively charged amino acids. The cleft is deep compared to more promiscuous serine proteases, consequently substrates must either have a large loop that is cleaved or have favorable interactions with the insertion loops <ref>PMID: 16102053</ref>. |
Many other loops project out of the B chain but most are rigid due to proline and tryptophan residues. | Many other loops project out of the B chain but most are rigid due to proline and tryptophan residues. | ||
| Line 66: | Line 66: | ||
'''Exosite I''' is located on the B chain and had both basic and hydrophobic character. It is important in binding <scene name='58/583418/Fibrinogen/1'>fibrinogen</scene>, platelet activated receptors, and <scene name='58/583418/Thrombomodulin/1'>thrombomodulin</scene>. | '''Exosite I''' is located on the B chain and had both basic and hydrophobic character. It is important in binding <scene name='58/583418/Fibrinogen/1'>fibrinogen</scene>, platelet activated receptors, and <scene name='58/583418/Thrombomodulin/1'>thrombomodulin</scene>. | ||
| - | '''Exosite II''' is also part of the B chain, and derived from numerous basic amino acids, this is the site of <scene name='58/583418/Antithrombin/1'>heparin binding</scene> through the sulfate groups on the glycosaminoglycan. It is also the site of | + | '''Exosite II''' is also part of the B chain, and derived from numerous basic amino acids, this is the site of <scene name='58/583418/Antithrombin/1'>heparin binding</scene> through the sulfate groups on the glycosaminoglycan. It is also the site of GpIbα binding on the platelet surface. |
The <scene name='58/583418/Sodium_binding_loop/1'>sodium binding site</scene> is formed by the 180s- and 220s- loops. Na+ is bound by the backbone oxygens of Arg221a and Lys224 in addition to four water molecules in a classic [http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Crystal_Field_Theory/High_Spin_and_Low_Spin_Complexes#Octahedral_Geometry octahedral geometry]<ref>PMID: 9108691</ref>. Through the covelent disulfide linkage between Cys220 and Cys 191 the sodium binding site is linked to Ser195 and the oxyanion hole. | The <scene name='58/583418/Sodium_binding_loop/1'>sodium binding site</scene> is formed by the 180s- and 220s- loops. Na+ is bound by the backbone oxygens of Arg221a and Lys224 in addition to four water molecules in a classic [http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Crystal_Field_Theory/High_Spin_and_Low_Spin_Complexes#Octahedral_Geometry octahedral geometry]<ref>PMID: 9108691</ref>. Through the covelent disulfide linkage between Cys220 and Cys 191 the sodium binding site is linked to Ser195 and the oxyanion hole. | ||
| Line 89: | Line 89: | ||
==Secondary Structure Features== | ==Secondary Structure Features== | ||
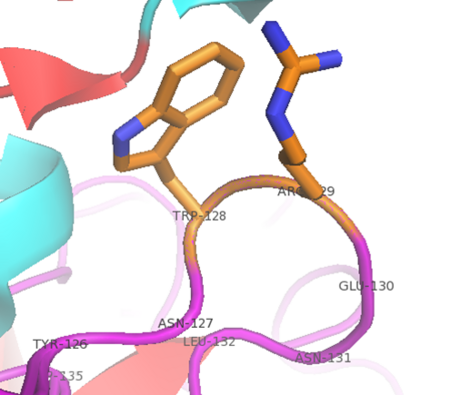
| - | Thrombin has a <scene name='58/583418/Cis_proline/1'>cis peptide bond</scene> in a loop containing proline 37. The dihedral ( | + | Thrombin has a <scene name='58/583418/Cis_proline/1'>cis peptide bond</scene> in a loop containing proline 37. The dihedral (C'-Cα-N-C') angle omega is -69.4 degrees. |
[[Image:Capping.png|450px|center|thumb| “Capping box” motif in alpha thrombin (PDB: 1PPB) represented by His230 (Ncap) side chain and main chain hydrogen bonded with the backbone nitrogen of Arg233 (N3). An additional feature is a weak hydrophobic interaction between Thr229 (N’) and Val234 (N4) termed the “hydrophobic staple.” This motif derives it’s name from the box shaped hydrogen bonding pattern.]] | [[Image:Capping.png|450px|center|thumb| “Capping box” motif in alpha thrombin (PDB: 1PPB) represented by His230 (Ncap) side chain and main chain hydrogen bonded with the backbone nitrogen of Arg233 (N3). An additional feature is a weak hydrophobic interaction between Thr229 (N’) and Val234 (N4) termed the “hydrophobic staple.” This motif derives it’s name from the box shaped hydrogen bonding pattern.]] | ||
Current revision
| |||||||||||
References
- ↑ Fenton JW 2nd. Thrombin specificity. Ann N Y Acad Sci. 1981;370:468-95. PMID:7023326
- ↑ 2.0 2.1 Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000 Sep 14;407(6801):258-64. PMID:11001069 doi:http://dx.doi.org/10.1038/35025229
- ↑ Crawley JT, Lam JK, Rance JB, Mollica LR, O'Donnell JS, Lane DA. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood. 2005 Feb 1;105(3):1085-93. Epub 2004 Sep 23. PMID:15388580 doi:http://dx.doi.org/10.1182/blood-2004-03-1101
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Lane DA, Philippou H, Huntington JA. Directing thrombin. Blood. 2005 Oct 15;106(8):2605-12. Epub 2005 Jun 30. PMID:15994286 doi:http://dx.doi.org/10.1182/blood-2005-04-1710
- ↑ Takagi T, Doolittle RF. Amino acid sequence studies on factor XIII and the peptide released during its activation by thrombin. Biochemistry. 1974 Feb 12;13(4):750-6. PMID:4811064
- ↑ Miljic P, Heylen E, Willemse J, Djordjevic V, Radojkovic D, Colovic M, Elezovic I, Hendriks D. Thrombin activatable fibrinolysis inhibitor (TAFI): a molecular link between coagulation and fibrinolysis. Srp Arh Celok Lek. 2010 Jan;138 Suppl 1:74-8. PMID:20229688
- ↑ 7.0 7.1 7.2 7.3 Huntington JA. Natural inhibitors of thrombin. Thromb Haemost. 2014 Apr 1;111(4):583-9. doi: 10.1160/TH13-10-0811. Epub 2014 Jan, 30. PMID:24477356 doi:http://dx.doi.org/10.1160/TH13-10-0811
- ↑ 8.0 8.1 8.2 8.3 8.4 Huntington JA. Thrombin inhibition by the serpins. J Thromb Haemost. 2013 Jun;11 Suppl 1:254-64. doi: 10.1111/jth.12252. PMID:23809129 doi:http://dx.doi.org/10.1111/jth.12252
- ↑ Esmon CT. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348-52. PMID:3029867
- ↑ Kalafatis M, Rand MD, Mann KG. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J Biol Chem. 1994 Dec 16;269(50):31869-80. PMID:7989361
- ↑ 11.0 11.1 Lu D, Kalafatis M, Mann KG, Long GL. Comparison of activated protein C/protein S-mediated inactivation of human factor VIII and factor V. Blood. 1996 Jun 1;87(11):4708-17. PMID:8639840
- ↑ Duga S, Asselta R, Tenchini ML. Coagulation factor V. Int J Biochem Cell Biol. 2004 Aug;36(8):1393-9. PMID:15147718 doi:http://dx.doi.org/10.1016/j.biocel.2003.08.002
- ↑ Saenko EL, Shima M, Sarafanov AG. Role of activation of the coagulation factor VIII in interaction with vWf, phospholipid, and functioning within the factor Xase complex. Trends Cardiovasc Med. 1999 Oct;9(7):185-92. PMID:10881749
- ↑ Camire, R. M. (2010). Platelet factor V to the rescue. Blood, 115(4), 753-754. DOI: 10.1182/blood-2009-11-252619
- ↑ Berkner KL. Vitamin K-dependent carboxylation. Vitam Horm. 2008;78:131-56. doi: 10.1016/S0083-6729(07)00007-6. PMID:18374193 doi:http://dx.doi.org/10.1016/S0083-6729(07)00007-6
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 Lechtenberg BC, Freund SM, Huntington JA. An ensemble view of thrombin allostery. Biol Chem. 2012 Sep;393(9):889-98. doi: 10.1515/hsz-2012-0178. PMID:22944689 doi:http://dx.doi.org/10.1515/hsz-2012-0178
- ↑ Tijburg PN, van Heerde WL, Leenhouts HM, Hessing M, Bouma BN, de Groot PG. Formation of meizothrombin as intermediate in factor Xa-catalyzed prothrombin activation on endothelial cells. The influence of thrombin on the reaction mechanism. J Biol Chem. 1991 Feb 25;266(6):4017-22. PMID:1995649
- ↑ Bobofchak KM, Pineda AO, Mathews FS, Di Cera E. Energetic and structural consequences of perturbing Gly-193 in the oxyanion hole of serine proteases. J Biol Chem. 2005 Jul 8;280(27):25644-50. Epub 2005 May 12. PMID:15890651 doi:http://dx.doi.org/10.1074/jbc.M503499200
- ↑ 19.0 19.1 19.2 19.3 Bode W, Mayr I, Baumann U, Huber R, Stone SR, Hofsteenge J. The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989 Nov;8(11):3467-75. PMID:2583108
- ↑ Page MJ, Di Cera E. Evolution of peptidase diversity. J Biol Chem. 2008 Oct 31;283(44):30010-4. doi: 10.1074/jbc.M804650200. Epub 2008 , Sep 3. PMID:18768474 doi:http://dx.doi.org/10.1074/jbc.M804650200
- ↑ Schechter I, Berger A. On the size of the active site in proteases. I. Papain. 1967. Biochem Biophys Res Commun. 2012 Aug 31;425(3):497-502. doi:, 10.1016/j.bbrc.2012.08.015. PMID:22925665 doi:http://dx.doi.org/10.1016/j.bbrc.2012.08.015
- ↑ Huntington JA. Molecular recognition mechanisms of thrombin. J Thromb Haemost. 2005 Aug;3(8):1861-72. PMID:16102053 doi:http://dx.doi.org/10.1111/j.1538-7836.2005.01363.x
- ↑ Zhang E, Tulinsky A. The molecular environment of the Na+ binding site of thrombin. Biophys Chem. 1997 Jan 31;63(2-3):185-200. PMID:9108691
- ↑ Li W, Johnson DJ, Esmon CT, Huntington JA. Structure of the antithrombin-thrombin-heparin ternary complex reveals the antithrombotic mechanism of heparin. Nat Struct Mol Biol. 2004 Sep;11(9):857-62. Epub 2004 Aug 15. PMID:15311269 doi:10.1038/nsmb811
- ↑ Spronk HM, Borissoff JI, ten Cate H. New insights into modulation of thrombin formation. Curr Atheroscler Rep. 2013 Nov;15(11):363. doi: 10.1007/s11883-013-0363-3. PMID:24026641 doi:http://dx.doi.org/10.1007/s11883-013-0363-3