Sandbox Reserved 933
From Proteopedia
| Line 19: | Line 19: | ||
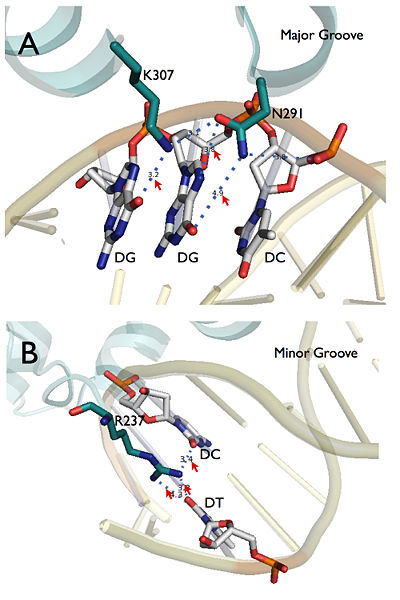
The general structure of LEAFY DNA binding domain consists 2 <scene name='57/579703/Beta-strand/1'>β strands</scene> at the beginning followed by 7 <scene name='57/579703/Alpha-helices_color/2'> α helices</scene>. A <scene name='57/579703/Alpha-helices_color/3'>helix-turn-helix</scene> (HTH) motif can be found between α2 and α3 helices, which is recruited to the <scene name='57/579703/Major_groove/2'>major groove </scene>of the binding DNA. There are two amino acid at this motif, <scene name='57/579703/Major_groove_asn291/1'>Asn 291</scene> on α2 and <scene name='57/579703/Major_groove_asn291/2'>Lys 307</scene> on α3 directly mediate site specific recognition with <scene name='57/579703/Major_groove_detail/1'>two guanines</scene> at the DNA strand. These two recognition sites were further validated by electrophoresis mobility shift assay (EMSA): mutation at either Asn 291 or Lys 307 dramatically decrease binding affinity to pAP1. In the minor groove, site specific recognition is conducted by <scene name='57/579703/Arg_237/1'>Arg 237</scene>, which is at the beginning of this structure. ''Arabidopsis'' intermediate mutant ''lfy-4'' (P240L) and ''lfy-5'' (T244M) were located near this site and validate the function ''in planta''<ref name="Weigel1992" />. The super position of specific recognition sites is summaries at figure 2. | The general structure of LEAFY DNA binding domain consists 2 <scene name='57/579703/Beta-strand/1'>β strands</scene> at the beginning followed by 7 <scene name='57/579703/Alpha-helices_color/2'> α helices</scene>. A <scene name='57/579703/Alpha-helices_color/3'>helix-turn-helix</scene> (HTH) motif can be found between α2 and α3 helices, which is recruited to the <scene name='57/579703/Major_groove/2'>major groove </scene>of the binding DNA. There are two amino acid at this motif, <scene name='57/579703/Major_groove_asn291/1'>Asn 291</scene> on α2 and <scene name='57/579703/Major_groove_asn291/2'>Lys 307</scene> on α3 directly mediate site specific recognition with <scene name='57/579703/Major_groove_detail/1'>two guanines</scene> at the DNA strand. These two recognition sites were further validated by electrophoresis mobility shift assay (EMSA): mutation at either Asn 291 or Lys 307 dramatically decrease binding affinity to pAP1. In the minor groove, site specific recognition is conducted by <scene name='57/579703/Arg_237/1'>Arg 237</scene>, which is at the beginning of this structure. ''Arabidopsis'' intermediate mutant ''lfy-4'' (P240L) and ''lfy-5'' (T244M) were located near this site and validate the function ''in planta''<ref name="Weigel1992" />. The super position of specific recognition sites is summaries at figure 2. | ||
=== DNA binding required cooperative dimerization === | === DNA binding required cooperative dimerization === | ||
| - | [[Image:Dimer_Bond.001.jpg|400px| | + | [[Image:Dimer_Bond.001.jpg|400px|right|thumb| Figure 3. Three residues mediate homodimerization of LFY dimerization at pAP1 site. Assembly of PDB entry 2VY1 were obtained from PISA server and further visualized by Pymol. ]] |
Transcription factors tent to form homodimer or heterodimer to increase the binding specificity and affinity. Experimental evidence indicates a potential LFY dimer on the binding site. Crystal structure proved that LFY can form dimers at both <scene name='57/579703/Ap1-dimer/2'>pAP1</scene> and <scene name='57/579703/2vy2_assembly/2'>pAG</scene> sites. The binding affinity of LFY protein dimer binds increased by 90-fold compared to the first LFY monomer in EMSA assay. Detailed structure revealed that the contact of two dimerized protein is mediated by <scene name='57/579703/Ap1-dimer/8'>three residues</scene> located at on helix (<scene name='57/579703/Ap1-dimer/3'>α7</scene>) and one loop (<scene name='57/579703/Ap1-dimer/4'>loop12</scene>) at the other protein. Hydrogen bonds can be formed between <scene name='57/579703/Ap1-dimer/7'>Asp 280</scene> and <scene name='57/579703/Ap1-dimer/5'>His 387</scene>/<scene name='57/579703/Ap1-dimer/6'>Arg 390</scene> are essential for this dimeriation. The detailed interaction is shown in figure 3 produced by Pymol. Mutation in any of these three amino acids abolished the binding in EMSA assay. Recently, another experiment showing that besides these three residues, the entire N-terminal consensus is critical important for stabilizing the homodimerization, where strong physical interaction can be found by GST-pull down, Y2H and BiFC experiment at ''in vitro'', ''in vivo'' and ''in planta'' level <ref name= ''Siriwardana2012'' > Siriwardana, N. S. & Lamb, R. S. 2012. A conserved domain in the N-terminus is important for LEAFY dimerization and function in Arabidopsis thaliana. The Plant Journal 71: 736–749. http://dx.doi.org/10.1111/j.1365-313X.2012.05026.x8</ref> . | Transcription factors tent to form homodimer or heterodimer to increase the binding specificity and affinity. Experimental evidence indicates a potential LFY dimer on the binding site. Crystal structure proved that LFY can form dimers at both <scene name='57/579703/Ap1-dimer/2'>pAP1</scene> and <scene name='57/579703/2vy2_assembly/2'>pAG</scene> sites. The binding affinity of LFY protein dimer binds increased by 90-fold compared to the first LFY monomer in EMSA assay. Detailed structure revealed that the contact of two dimerized protein is mediated by <scene name='57/579703/Ap1-dimer/8'>three residues</scene> located at on helix (<scene name='57/579703/Ap1-dimer/3'>α7</scene>) and one loop (<scene name='57/579703/Ap1-dimer/4'>loop12</scene>) at the other protein. Hydrogen bonds can be formed between <scene name='57/579703/Ap1-dimer/7'>Asp 280</scene> and <scene name='57/579703/Ap1-dimer/5'>His 387</scene>/<scene name='57/579703/Ap1-dimer/6'>Arg 390</scene> are essential for this dimeriation. The detailed interaction is shown in figure 3 produced by Pymol. Mutation in any of these three amino acids abolished the binding in EMSA assay. Recently, another experiment showing that besides these three residues, the entire N-terminal consensus is critical important for stabilizing the homodimerization, where strong physical interaction can be found by GST-pull down, Y2H and BiFC experiment at ''in vitro'', ''in vivo'' and ''in planta'' level <ref name= ''Siriwardana2012'' > Siriwardana, N. S. & Lamb, R. S. 2012. A conserved domain in the N-terminus is important for LEAFY dimerization and function in Arabidopsis thaliana. The Plant Journal 71: 736–749. http://dx.doi.org/10.1111/j.1365-313X.2012.05026.x8</ref> . | ||
</StructureSection> | </StructureSection> | ||
Revision as of 03:22, 19 May 2014
| This Sandbox is Reserved from 01/04/2014, through 30/06/2014 for use in the course "510042. Protein structure, function and folding" taught by Prof Adrian Goldman, Tommi Kajander, Taru Meri, Konstantin Kogan and Juho Kellosalo at the University of Helsinki. This reservation includes Sandbox Reserved 923 through Sandbox Reserved 947. |
To get started:
More help: Help:Editing |
Contents |
Evolution of DNA binding domain of LEAFY: from angiosperms to mosses
Introduction
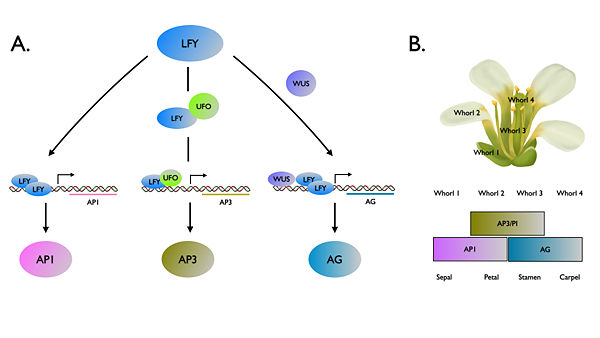
FLORICAULA/LEAFY (FLO/LFY) genes encode a plant specific transcription factor family that controlling floral fate of reproductive phase. [1][2]. In the plant model system Arabidopsis thaliana , ‘’LFY’’ also acts upstream of floral homeotic genes to modulate floral organ identity. [3] LFY activates the downstream genes by binding to promoter regions. LFY can directly bind to the promoter to APELATA1 (AP1), while co-regulators UNUSUAL FLORAL ORGANS (UFO) [4] and WUSCHEL (WUS)[5] are required for increment of binding affinity to promoter regions of APELATA3 (AP3) and AGAMOUS (AG), respectively (Figure 1). The exact mechanism how LFY recognizing and binding to these promoters has yet to be elucidated until the first structure report about two DNA-protein complex: and [6] . Among land plants, FLO/LFY homologs share a highly conserved DNA binding region that a hypothesis claimed substitution in this domain might result in the functional divergence[7] . Recently, a new structure about LFY homolog in mosses provided new insights of structural basis of how LEAFY evolved by changing DNA binding activity[8].
| |||||||||||
LEAFY Evolution
Different from other transcription factor families, LFY and its homologs retains to be a single copy gene in almost all land plants. This brought a new entry point that how LFY evolved to control different developmental processes in other plant lineages. Multiple alignment of LFY and its homologs revealed that at specific position of DNA binding domain, few amino acids were substituted from angiosperms to algae.
Reference
- ↑ 1.0 1.1 1.2 Weigel, D., Alvarez, J., David R., Yanofsky, M.F. & Meyerowitz, E.M. 1992. LEAFY controls floral meristem identity in Arabidopsis. Cell 69 :843-859, http://dx.doi.org/10.1016/0092-8674(92)90295-N.
- ↑ 2.0 2.1 Coen, E.S., Romero, J.M., Doyle, S., Elliot, R., Murphy, G. & Carpenter, R. 1990. Floricaula: a homeotic gene required for flower development in Antirrhinum majus. Cell 63: 1311–1322 http://dx.doi.org/10.1016/0092-8674(90)90426-F
- ↑ Irish, V. F. 2010. The flowering of Arabidopsis flower development. The Plant Journal, 61: 1014–1028. http://dx.doi.org/10.1111/j.1365-313X.2009.04065.x
- ↑ Chae, E., Tan, Q.K., Hill, T.A. & Irish, V.F. 2008. An Arabidopsis F-box protein acts as a transcriptional co-factor to regulate floral development. Development 135:1235-45 http://dx.doi.org/10.1242/dev.015842
- ↑ HONG, R.L., HAMAGUCHI, L., BUSCH, M.A. and WEIGEL, D. 2003. Regulatory elements of the floral homeotic gene AGAMOUS identified by phylogenetic footprinting and shadowing. The Plant Cell 15: 1296-1309. http://dx.doi.org/10.1105/tpc.009548
- ↑ Hames, C., Ptchelkine, D., Grimm, C., Thevenon, E., Moyroud, E., Gérard, F. Martiel, J.L., Benlloch, R., Parcy, F. & Müller, C.W. 2008. Structural basis for LEAFY floral switch function and similarity with helix-turn-helix proteins. EMBO Journal 27:2628-2637. http://dx.doi.org/10.1038/emboj.2008.184
- ↑ MAIZEL, A., BUSCH, M.A., TANAHASHI, T., PERKOVIC, J., KATAO, M., HASEBE, M. and WEIGEL, D. (2005). The floral regulator LEAFY evolves by substitutions in the DNA binding domain. Science 308: 260-263. http://dx.doi.org/10.1126/science.1108229
- ↑ Sayou, C., Monniaux, M., Nanao, M.H., Moyroud, E., Brockington, S.F., Thévenon, E., Chahtane, H., Warthmann, N., Melkonian, M., Zhang, Y., Wong, G., Weigel, D., Parcy, F. and Dumas, R. 2014. A Promiscuous Intermediate Underlies the Evolution of LEAFY DNA Binding Specificity Science 343: 645-648 http://dx.doi.org/10.1126/science.1248229
- ↑ Siriwardana, N. S. & Lamb, R. S. 2012. A conserved domain in the N-terminus is important for LEAFY dimerization and function in Arabidopsis thaliana. The Plant Journal 71: 736–749. http://dx.doi.org/10.1111/j.1365-313X.2012.05026.x8