We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
4d2c
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
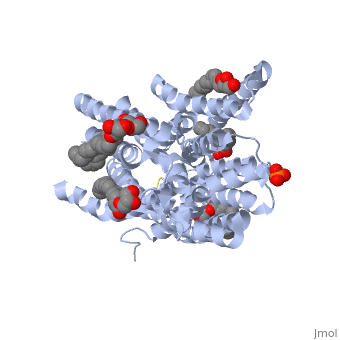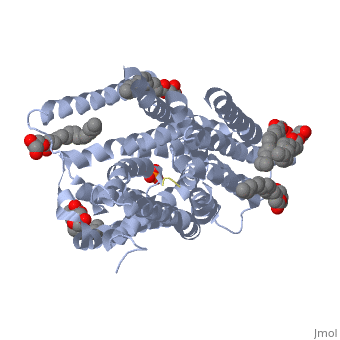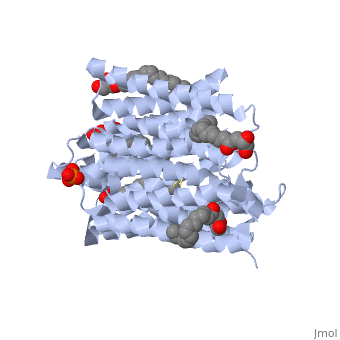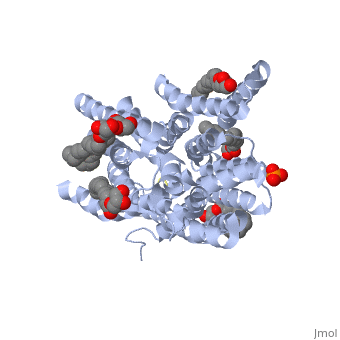
| - | ''' | + | ==Structure of a di peptide bound POT family peptide transporter== |
| + | <StructureSection load='4d2c' size='340' side='right' caption='[[4d2c]], [[Resolution|resolution]] 2.47Å' scene=''> | ||
| + | == Structural highlights == | ||
| + | <table><tr><td colspan='2'>[[4d2c]] is a 2 chain structure. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=4D2C OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4D2C FirstGlance]. <br> | ||
| + | </td></tr><tr><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=78M:(2S)-2,3-DIHYDROXYPROPYL(7Z)-PENTADEC-7-ENOATE'>78M</scene>, <scene name='pdbligand=78N:(2R)-2,3-DIHYDROXYPROPYL(7Z)-PENTADEC-7-ENOATE'>78N</scene>, <scene name='pdbligand=PO4:PHOSPHATE+ION'>PO4</scene><br> | ||
| + | <tr><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[4d2b|4d2b]], [[4d2d|4d2d]]</td></tr> | ||
| + | <tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4d2c FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4d2c OCA], [http://www.rcsb.org/pdb/explore.do?structureId=4d2c RCSB], [http://www.ebi.ac.uk/pdbsum/4d2c PDBsum]</span></td></tr> | ||
| + | <table> | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Publication Abstract from PubMed == | ||
| + | An enigma in the field of peptide transport is the structural basis for ligand promiscuity, as exemplified by PepT1, the mammalian plasma membrane peptide transporter. Here, we present crystal structures of di- and tripeptide-bound complexes of a bacterial homologue of PepT1, which reveal at least two mechanisms for peptide recognition that operate within a single, centrally located binding site. The dipeptide was orientated laterally in the binding site, whereas the tripeptide revealed an alternative vertical binding mode. The co-crystal structures combined with functional studies reveal that biochemically distinct peptide-binding sites likely operate within the POT/PTR family of proton-coupled symporters and suggest that transport promiscuity has arisen in part through the ability of the binding site to accommodate peptides in multiple orientations for transport. | ||
| - | + | Structural basis for polyspecificity in the POT family of proton-coupled oligopeptide transporters.,Lyons JA, Parker JL, Solcan N, Brinth A, Li D, Shah ST, Caffrey M, Newstead S EMBO Rep. 2014 Jun 10. pii: e201338403. PMID:24916388<ref>PMID:24916388</ref> | |
| - | + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |
| - | + | </div> | |
| - | + | == References == | |
| + | <references/> | ||
| + | __TOC__ | ||
| + | </StructureSection> | ||
| + | [[Category: Brinth, A.]] | ||
| + | [[Category: Caffrey, M.]] | ||
| + | [[Category: Li, D.]] | ||
| + | [[Category: Lyons, J A.]] | ||
| + | [[Category: Newstead, S.]] | ||
| + | [[Category: Parker, J L.]] | ||
| + | [[Category: Shah, S T.A.]] | ||
| + | [[Category: Solcan, N.]] | ||
| + | [[Category: Major facilitator superfamily]] | ||
| + | [[Category: Peptide complex]] | ||
| + | [[Category: Peptide transporter]] | ||
| + | [[Category: Transport protein]] | ||
Revision as of 06:27, 25 June 2014
Structure of a di peptide bound POT family peptide transporter
| |||||||||||