Aconitase
From Proteopedia
| Line 1: | Line 1: | ||
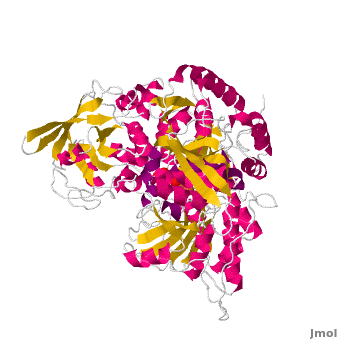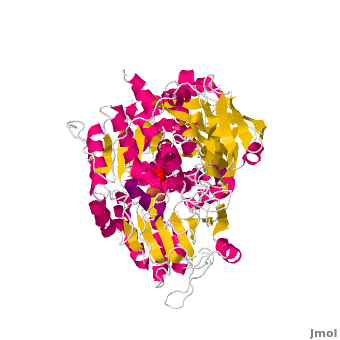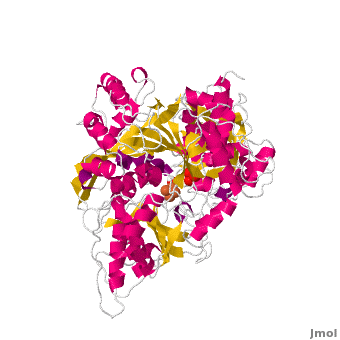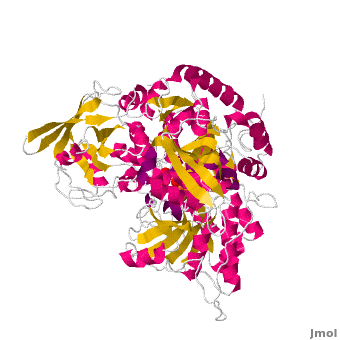
<StructureSection load='' size='450' side='right' scene='Aconitase/Cv/1' caption='Bovine aconitase showing FeS4 cluster complex with sulfate (PDB code [[1amj]])'> | <StructureSection load='' size='450' side='right' scene='Aconitase/Cv/1' caption='Bovine aconitase showing FeS4 cluster complex with sulfate (PDB code [[1amj]])'> | ||
| - | + | ||
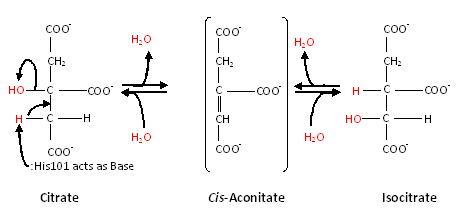
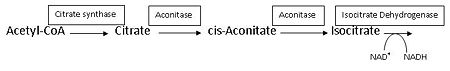
[[Aconitase]] (ACO, EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=4.2.1.3 4.2.1.3]) is an enzymatic domain that confers the ability to catalyse the equilibrium | [[Aconitase]] (ACO, EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=4.2.1.3 4.2.1.3]) is an enzymatic domain that confers the ability to catalyse the equilibrium | ||
:citrate = aconitate + H<sub>2</sub>O = L-isocitrate | :citrate = aconitate + H<sub>2</sub>O = L-isocitrate | ||
Revision as of 06:31, 24 August 2014
| |||||||||||
Contents |
3D structures of Aconitase
Updated on 24-August-2014
ACO
1b0k – pACO (mutant) – pig
5acn – pACO+Fe3S4
6acn - pACO+Fe4S4
1amj, 1nit – cACO - cow
ACO+citrate
1c96 - pACO (mutant)+citrate
1b0m - pACO (mutant)+fluorocitrate
ACO+aconitate
1fgh – cACO+4-hydroxy-aconitate
1aco – cACO+transaconitate
1nis - cACO+transaconitate+nitrocitrate
ACO+isocitrate
7acn - pACO +isocitrate
1c97, 1b0j - pACO (mutant)+isocitrate
1ami, 8acn – cACO+isocitrate
ACO1
2b3x, 2b3y – hACO1 – human
2ipy, 3snp – rACO1 (mutant)+ferritin H IRE-RNA – rabbit
3sn2 - rACO1 (mutant)+ transferrin receptor iron regulatory RNA
ACO2
1l5j – ACO2 – Escherichia coli
Literature
- M. Claire Kennedy and Helmut Beinert: IX.4. Aconitase. in Ivano Bertini, Harry B. Gray, Edward I. Stiefel, Joan Selverstone Valentine (eds.): Biological Inorganic Chemistry: Structure and Reactivity. University Science Books, Herndon 2006. ISBN 1891389432 pp.209--
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ Zheng L, Kennedy MC, Beinert H, Zalkin H. Mutational analysis of active site residues in pig heart aconitase. J Biol Chem. 1992 Apr 15;267(11):7895-903. PMID:1313811
- ↑ 2.0 2.1 Frishman D, Hentze MW. Conservation of aconitase residues revealed by multiple sequence analysis. Implications for structure/function relationships. Eur J Biochem. 1996 Jul 1;239(1):197-200. PMID:8706708
- ↑ Dupuy J, Volbeda A, Carpentier P, Darnault C, Moulis JM, Fontecilla-Camps JC. Crystal structure of human iron regulatory protein 1 as cytosolic aconitase. Structure. 2006 Jan;14(1):129-39. PMID:16407072 doi:10.1016/j.str.2005.09.009
- ↑ 4.0 4.1 4.2 Beinert, H., Kennedy, M. C., Stout, C.D. “Aconitase as Iron−Sulfur Protein, Enzyme, and Iron-Regulatory Protein.” Chem. Rev. 1996, 96, 2335−2373.
- ↑ Lauble H, Kennedy MC, Beinert H, Stout CD. Crystal structures of aconitase with trans-aconitate and nitrocitrate bound. J Mol Biol. 1994 Apr 8;237(4):437-51. PMID:8151704 doi:http://dx.doi.org/10.1006/jmbi.1994.1246
- ↑ 6.0 6.1 6.2 6.3 Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry Life at the Molecular Level. New York: John Wiley & Sons, 2008. p. 578-579. Print.
- ↑ 7.0 7.1 Flint, DH., and Allen, RM. "Iron-sulfur protein with nonredox functions.” Chem. Rev. 1996, 96, 2315−2334.
External links
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Ralf Stephan, David Canner, Joel L. Sussman, Jaime Prilusky, Anthony Noles, Angel Herraez, Eran Hodis