Lotem haleva/test page
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
==Trypsin== | ==Trypsin== | ||
<StructureSection load='1y3v' size='340' side='right' caption='Trysine' scene=''> | <StructureSection load='1y3v' size='340' side='right' caption='Trysine' scene=''> | ||
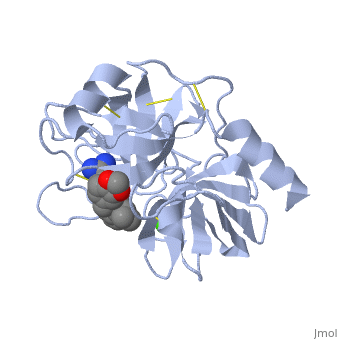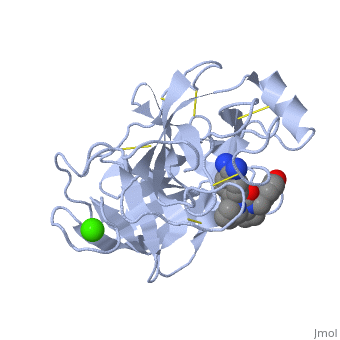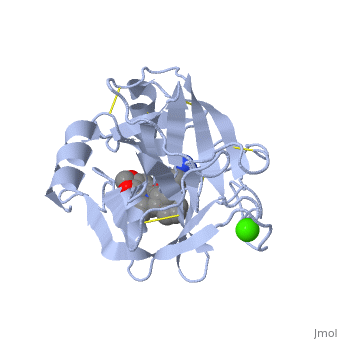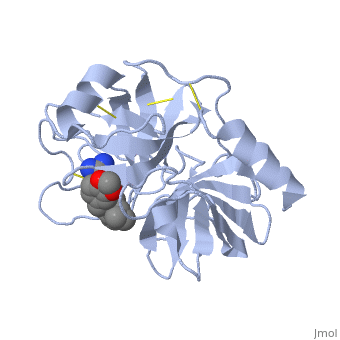
| - | Trypsin is a serine protease | + | Trypsin is a serine protease that found in the digestive system of many vertebrates, where it hydrolyses proteins<ref>doi:10.1016/0076-6879(94)44004-2</ref>. |
Trypsin is produced in the pancreas as the inactive protease trypsinogen. | Trypsin is produced in the pancreas as the inactive protease trypsinogen. | ||
| - | Trypsin Break peptide chains | + | Trypsin Break peptide chains at the carboxyl side of the amino acids lysine or arginine, |
except when it is followed by proline. . | except when it is followed by proline. . | ||
| Line 10: | Line 10: | ||
== Structure and Mechanism == | == Structure and Mechanism == | ||
| - | Trypsin is a medium size globular protein that contains either <scene name='60/607865/Helix/1'>alpha helix</scene> | + | Trypsin is a medium size globular protein that contains either <scene name='60/607865/Helix/1'>alpha helix</scene> and <scene name='60/607865/Sheets/1'>beta sheets</scene>. |
The enzymatic mechanism of Trypsin is similar to the other serine proteases. These enzymes contain a catalytic triad consisting of <scene name='60/607865/Active_site/2'>histidine-57, aspartate-102, and serine-195</scene>, These three residues form a charge relay that serves to make the active site serine nucleophilic <ref>doi:10.1007/s00018-005-5160-x</ref>. | The enzymatic mechanism of Trypsin is similar to the other serine proteases. These enzymes contain a catalytic triad consisting of <scene name='60/607865/Active_site/2'>histidine-57, aspartate-102, and serine-195</scene>, These three residues form a charge relay that serves to make the active site serine nucleophilic <ref>doi:10.1007/s00018-005-5160-x</ref>. | ||
Revision as of 11:48, 23 November 2014
Trypsin
| |||||||||||
References
- ↑ Rawlings ND, Barrett AJ. Families of serine peptidases. Methods Enzymol. 1994;244:19-61. doi: 10.1016/0076-6879(94)44004-2. PMID:7845208 doi:http://dx.doi.org/10.1016/0076-6879(94)44004-2
- ↑ Polgar L. The catalytic triad of serine peptidases. Cell Mol Life Sci. 2005 Oct;62(19-20):2161-72. PMID:16003488 doi:http://dx.doi.org/10.1007/s00018-005-5160-x
- ↑ Rodriguez J, Gupta N, Smith RD, Pevzner PA. Does trypsin cut before proline? J Proteome Res. 2008 Jan;7(1):300-5. Epub 2007 Dec 8. PMID:18067249 doi:http://dx.doi.org/10.1021/pr0705035