Lotem haleva/test page
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='1y3v' size='340' side='right' caption='Trysine' scene=''> | <StructureSection load='1y3v' size='340' side='right' caption='Trysine' scene=''> | ||
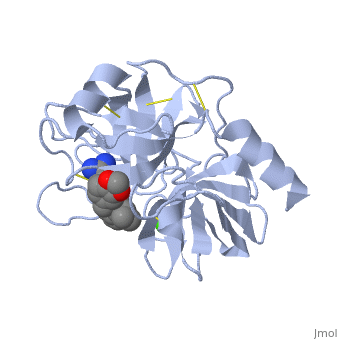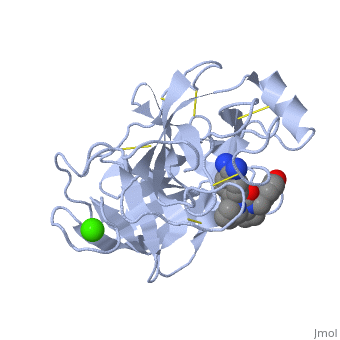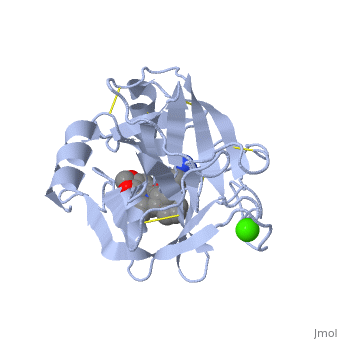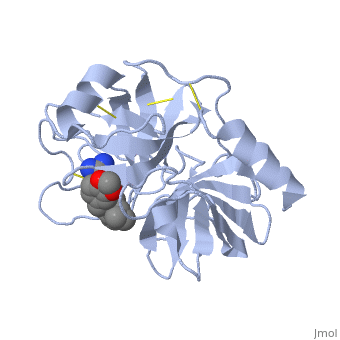
Trypsin is a serine protease that found in the digestive system of many vertebrates, where it hydrolyses proteins<ref>doi:10.1016/0076-6879(94)44004-2</ref>. | Trypsin is a serine protease that found in the digestive system of many vertebrates, where it hydrolyses proteins<ref>doi:10.1016/0076-6879(94)44004-2</ref>. | ||
| - | Trypsin is produced in the pancreas as the inactive protease trypsinogen. | + | Trypsin is produced in the pancreas as the inactive zymogen protease called trypsinogen. trypsinogen secreted into the small intestine where it activates trypsinogen into trypsin by the enzyme Antrookinaz using proteolytic decomposition. |
Trypsin Break peptide chains at the carboxyl side of the amino acids lysine or arginine, | Trypsin Break peptide chains at the carboxyl side of the amino acids lysine or arginine, | ||
except when it is followed by proline. . | except when it is followed by proline. . | ||
Revision as of 12:02, 23 November 2014
Trypsin
| |||||||||||
References
- ↑ Rawlings ND, Barrett AJ. Families of serine peptidases. Methods Enzymol. 1994;244:19-61. doi: 10.1016/0076-6879(94)44004-2. PMID:7845208 doi:http://dx.doi.org/10.1016/0076-6879(94)44004-2
- ↑ Polgar L. The catalytic triad of serine peptidases. Cell Mol Life Sci. 2005 Oct;62(19-20):2161-72. PMID:16003488 doi:http://dx.doi.org/10.1007/s00018-005-5160-x
- ↑ Rodriguez J, Gupta N, Smith RD, Pevzner PA. Does trypsin cut before proline? J Proteome Res. 2008 Jan;7(1):300-5. Epub 2007 Dec 8. PMID:18067249 doi:http://dx.doi.org/10.1021/pr0705035