AChE inhibitors and substrates
From Proteopedia
(Difference between revisions)
| Line 83: | Line 83: | ||
MB is a noncompetitive inhibitor of ''Tc''AChE, competing with reversible inhibitors directed at both ‘‘anionic’’ subsites, but a single site is involved in inhibition. The crystal structure reveals a <scene name='Journal:Protein_Science:1/Cv1/2'>single MB stacked against Trp279 in the PAS</scene>, oriented down the gorge toward the CAS ([[2w9i]]); it is plausible that irreversible inhibition is associated with photooxidation of this residue and others within the active-site gorge. Superposition of the '''PAS regions''' of the <font color='darkmagenta'><b>MB</b></font>/''Tc''AChE ([[2w9i]]) and <font color='magenta'><b>thioflavin T</b></font>/''Tc''AChE ([[2j3q]]) complexes reveals <scene name='Journal:Protein_Science:1/Cv1/4'>similarity between positions of these ligands</scene>. As the conformation of ''Tc''AChE in the crystal structures of the two complexes is practically identical, only that of the <font color='darkmagenta'><b>MB</b></font>/''Tc''AChE structure ([[2w9i]]) is shown. The kinetic and spectroscopic data showing that inhibitors binding at the '''CAS''' can impede binding of MB are reconciled by docking studies showing that the <scene name='Journal:Protein_Science:1/Cv2/5'>conformation adopted by Phe330</scene>, midway down the gorge, in the MB/''Tc''AChE crystal structure, precludes simultaneous binding of a second MB at the CAS (<font color='blueviolet'><b>2nd MB is colored blueviolet</b></font>, <span style="color:orange;background-color:black;font-weight:bold;">Phe330 of the crystal structure is in orange</span> and <font color='indigo'><b>Phe330 of the modeled structure is in indigo</b></font>). Conversely, binding of ligands at the '''CAS''' dislodges MB from its preferred locus at the '''PAS'''. The data presented demonstrate that TcAChE is a valuable model for understanding the molecular basis of local photooxidative damage.<ref name="Paz">PMID:22674800</ref> | MB is a noncompetitive inhibitor of ''Tc''AChE, competing with reversible inhibitors directed at both ‘‘anionic’’ subsites, but a single site is involved in inhibition. The crystal structure reveals a <scene name='Journal:Protein_Science:1/Cv1/2'>single MB stacked against Trp279 in the PAS</scene>, oriented down the gorge toward the CAS ([[2w9i]]); it is plausible that irreversible inhibition is associated with photooxidation of this residue and others within the active-site gorge. Superposition of the '''PAS regions''' of the <font color='darkmagenta'><b>MB</b></font>/''Tc''AChE ([[2w9i]]) and <font color='magenta'><b>thioflavin T</b></font>/''Tc''AChE ([[2j3q]]) complexes reveals <scene name='Journal:Protein_Science:1/Cv1/4'>similarity between positions of these ligands</scene>. As the conformation of ''Tc''AChE in the crystal structures of the two complexes is practically identical, only that of the <font color='darkmagenta'><b>MB</b></font>/''Tc''AChE structure ([[2w9i]]) is shown. The kinetic and spectroscopic data showing that inhibitors binding at the '''CAS''' can impede binding of MB are reconciled by docking studies showing that the <scene name='Journal:Protein_Science:1/Cv2/5'>conformation adopted by Phe330</scene>, midway down the gorge, in the MB/''Tc''AChE crystal structure, precludes simultaneous binding of a second MB at the CAS (<font color='blueviolet'><b>2nd MB is colored blueviolet</b></font>, <span style="color:orange;background-color:black;font-weight:bold;">Phe330 of the crystal structure is in orange</span> and <font color='indigo'><b>Phe330 of the modeled structure is in indigo</b></font>). Conversely, binding of ligands at the '''CAS''' dislodges MB from its preferred locus at the '''PAS'''. The data presented demonstrate that TcAChE is a valuable model for understanding the molecular basis of local photooxidative damage.<ref name="Paz">PMID:22674800</ref> | ||
{{Clear}} | {{Clear}} | ||
| + | |||
| + | ==AChE bivalent inhibitors== | ||
====OTMA==== | ====OTMA==== | ||
OTMA is a nonhydrolyzable [http://en.wikipedia.org/wiki/Substrate_analog substrate analogue] of AChE. Its [http://en.wikipedia.org/wiki/Hydrolysis hydrolysis] is impossible as <scene name='2vja/Common/3'>OTMA</scene> possesses <scene name='2vja/Common/4'>carbon</scene> atom instead of the <scene name='2vja/Common/5'>ester oxygen</scene> in the AChE natural substrate ACh. Similarly to ACh, OTMA covalently binds to the ''Tc''AChE ([[2vja]]) <scene name='2vja/Active_site/1'>Ser200</scene> Oγ at the CAS. At this subsite OTMA also interacts with <scene name='2vja/Active_site/2'>Trp84, Phe330</scene> ([http://en.wikipedia.org/wiki/Cation-pi_interaction cation-π interactions]); <scene name='2vja/Active_site/3'>Glu199</scene> (electrostatic interaction); <scene name='2vja/Active_site/4'>Gly118, Gly119, and Ala201</scene> (hydrogen bonds). OTMA binds not only at CAS, but also at PAS. A second OTMA molecule interacts with <scene name='2vja/Active_site/5'>Trp279, Tyr70</scene> (cation-π interactions), and <scene name='2vja/Active_site/6'>Tyr121</scene> (weak hydrogen bond) <ref name="Colletier">PMID:18701720</ref>. Thus, this dual binding mode of OTMA with ''Tc''AChE (to CAS and PAS) could be prototypical for [[AChE bivalent inhibitors]]. | OTMA is a nonhydrolyzable [http://en.wikipedia.org/wiki/Substrate_analog substrate analogue] of AChE. Its [http://en.wikipedia.org/wiki/Hydrolysis hydrolysis] is impossible as <scene name='2vja/Common/3'>OTMA</scene> possesses <scene name='2vja/Common/4'>carbon</scene> atom instead of the <scene name='2vja/Common/5'>ester oxygen</scene> in the AChE natural substrate ACh. Similarly to ACh, OTMA covalently binds to the ''Tc''AChE ([[2vja]]) <scene name='2vja/Active_site/1'>Ser200</scene> Oγ at the CAS. At this subsite OTMA also interacts with <scene name='2vja/Active_site/2'>Trp84, Phe330</scene> ([http://en.wikipedia.org/wiki/Cation-pi_interaction cation-π interactions]); <scene name='2vja/Active_site/3'>Glu199</scene> (electrostatic interaction); <scene name='2vja/Active_site/4'>Gly118, Gly119, and Ala201</scene> (hydrogen bonds). OTMA binds not only at CAS, but also at PAS. A second OTMA molecule interacts with <scene name='2vja/Active_site/5'>Trp279, Tyr70</scene> (cation-π interactions), and <scene name='2vja/Active_site/6'>Tyr121</scene> (weak hydrogen bond) <ref name="Colletier">PMID:18701720</ref>. Thus, this dual binding mode of OTMA with ''Tc''AChE (to CAS and PAS) could be prototypical for [[AChE bivalent inhibitors]]. | ||
| + | |||
| + | ===Tacrine- and hupyridone-containing compounds=== | ||
| + | The <scene name='1zgb/Act_site/3'>active site</scene> of ''Tc''AChE consists of two binding subsites. First of them is the "catalytic anionic site" (CAS), which involves mentioned above [http://en.wikipedia.org/wiki/Catalytic_triad catalytic triad] <scene name='1zgb/Act_site/8'>Ser200, His440, and Glu327</scene> <font color='orange'><b>(colored orange)</b></font> and the [http://en.wikipedia.org/wiki/Conserved_sequence#Conserved_protein_sequences_and_Structures conserved residues] <scene name='1zgb/Act_site/5'>Trp84</scene> and <scene name='1zgb/Act_site/10'>Phe330</scene> also participating in ligands recognition. Another conserved residue <scene name='1zgb/Act_site/11'>Trp279</scene> <font color='cyan'><b>(colored cyan)</b></font> is situated at the second binding subsite, termed the "peripheral anionic site" (PAS), ~14 Å from CAS. Therefore, the ligands that will be able to interact with both these subsites, will be more potent [http://en.wikipedia.org/wiki/Acetylcholinesterase_inhibitor AChE inhibitors] in comparison to compounds interacting only with CAS. One of the ways to produce such ligands is to introduce two active substances into one compound. If it is spatially necessary these subunits could be divided by alkyl linker with suitable length. | ||
| + | |||
| + | ====(''RS'')-(±)-tacrine-(10)-hupyridone==== | ||
| + | |||
| + | According to the strategy of the use of a bivalent ligand, the <scene name='1zgb/Comp/7'>inhibitor</scene> '''(''RS'')-(±)-tacrine-(10)-hupyridone''' ((R)-3 or (S)-3) was designed and synthesized. It consists of mentioned in the page '[[AChE inhibitors and substrates]]' <scene name='1zgb/Comp/8'>tacrine</scene> <font color='magenta'><b>(colored magenta)</b></font>, 10-carbon <scene name='1zgb/Comp/9'>linker</scene> <font color='yellow'><b>(yellow)</b></font>, and <scene name='1zgb/Comp/10'>hupyridone</scene> <font color='red'><b>(red)</b></font>. The tacrine moiety of this inhibitor binds at the CAS, the linker spans the <scene name='1zgb/Act_site/12'>active-site</scene> gorge, and the hupyridone moiety binds at the PAS. | ||
| + | The comparison of the (R)-3/''Tc''AChE and tacrine/''Tc''AChE complexes at the <scene name='1zgb/Align/2'>active site</scene>. A <scene name='1zgb/Align/7'>comparison</scene> of the trigonal (R)-3/''Tc''AChE structure ([[1zgb]]; <font color='cyan'><b>(R)-3 colored cyan</b></font>; ''Tc''AChE residues interacting with (R)-3 are colored sea-green) with the crystal structure of tacrine/''Tc''AChE ([[1acj]], <font color='magenta'><b>tacrine colored magenta</b></font>; residues interacting with [http://en.wikipedia.org/wiki/Tacrine tacrine] are colored <font color='pink'><b>pink</b></font>) reveals a similar binding mode for the tacrine moiety. In both structures the tacrine ring is situated at the CAS, between the [http://en.wikipedia.org/wiki/Aromaticity aromatic] residues Trp84 and Phe330. Steric clash with the 10-carbon linker could explain the tilt observed for the Phe330 <font color='yellow'><b> (yellow</b></font> and transparent in the tacrine/''Tc''AChE). <font color='red'><b>Water molecules are shown as red spheres.</b></font> | ||
| + | The tacrine unit of (R)-3 N forms <scene name='1zgb/Align/8'>hydrogen bond</scene> with His440 O (3.0 Å) similar to that of tacrine alone. Similarly to the tacrine/''Tc''AChE structure the <font color='red'><b>system of three water molecules</b></font> at the CAS ((R)-3/''Tc''AChE) binds the tacrine-linker N via hydrogen bonds to Ser81 O, Ser122 Oγ, and Asn85 Oδ1 (2.6-3.5 Å). | ||
| + | The <scene name='1zgc/Al/5'>overlap</scene> of <font color='cyan'><b>(R)-3 (cyan)</b></font> and <font color='orange'><b>(S)-3</b></font> ([[1zgc]]) bound to the ''Tc''AChE active site in the orthorhombic forms is shown. <scene name='1zgc/Zgc_h22/2'>Superposition</scene> of <font color='magenta'><b>(S,S)-(-)-4a (magenta)</b></font> and <font color='orange'><b>(S)-3 (orange, orthorhombic ''Tc''AChE)</b></font> demonstrates the similar mode of binding of the hupyridone unit at the PAS. The residues Trp279 (top) and Trp84 (bottom) represent the PAS and the CAS, respectively <ref name="Haviv">PMID:16076210</ref>. | ||
| + | |||
| + | {{Clear}} | ||
| + | |||
| + | ====Bis-hupyridone==== | ||
| + | The comparison of the (R)-3/''Tc''AChE ([[1zgb]]) and bis-hupyridone/''Tc''AChE complexes ([[1h22]] and [[1h23]]) at the <scene name='1zgb/Align2/2'>active site</scene>. <scene name='1zgb/Align2/8'>Superposition</scene> of the <font color='cyan'><b>(''R'')-tacrine-(10)-hupyridone ((R)-3, cyan)</b></font> and <font color='orange'><b>(S,S)-(-)-''Bis''(12)-hupyridone ('''(S,S)-(-)-4b''', orange, ''i.e.'' 12-carbon-tether-linked hupyridone dimer)</b></font> and <font color='plum'><b>(S,S)-(-)-''Bis''(10)-hupyridone ('''(S,S)-(-)-4a''', plum)</b></font> complexes demonstrates the binding mode of the hupyridone moiety. <font color='magenta'><b>TcAChE residues of symmetry-related molecule are shown in magenta.</b></font> X-ray structures of ''Tc''AChE complexed with these 10- and 12-carbon-tether-linked 2 <scene name='1zgb/Align2/9'>dimers</scene> <font color='plum'><b>(S,S)-(-)-4a</b></font> and <font color='orange'><b>(S,S)-(-)-4b</b></font> show one subunit bound at the <scene name='1zgb/Align2/10'>CAS</scene>, the linker spanning the gorge, and the other subunit bound at the <scene name='1zgb/Align2/11'>PAS</scene>. | ||
| + | There are two <scene name='1zgb/Align2/12'>hydrogen bonds</scene> connecting the <font color='cyan'><b>hupyridone</b></font> <font color='red'><b>O</b></font> to <font color='magenta'><b>Lys11</b></font> <font color='blue'><b>Nζ</b></font> and <font color='cyan'><b>hupyridone</b></font> <font color='blue'><b>N</b></font> to <font color='magenta'><b>Gln185</b></font> <font color='red'><b>Oε1</b></font> of a <font color='magenta'><b>symmetry-related molecule</b></font> at <font color='cyan'><b>(R)-3</b></font>/''Tc''AChE complex. <font color='red'><b>Water molecules are shown as red spheres.</b></font> Another hydrogen bond connects the <font color='cyan'><b>hupyridone</b></font> <font color='red'><b>O</b></font> to a water molecule, which is bound to Ser286 N. Similarly, the hupyridone-PAS unit of both <font color='plum'><b>(-)-4a</b></font> and <font color='orange'><b>(-)-4b</b></font> forms direct and an indirect hydrogen bonds with the protein backbone in the PAS region <ref name="Haviv"/> <ref name="Wong">PMID:12517147</ref>. | ||
| + | {{Clear}} | ||
| + | |||
| + | |||
| + | ====Bis(''n'')-tacrine derivatives==== | ||
| + | <font color='magenta'><b>2d</b></font> and <font color='orange'><b>2f</b></font> are bis(''n'')-tacrine derivatives with n=5 and 7 (number of carbons in the linkers), respectively. These compounds are more potent and selective AChE inhibitors than tacrine alone. The binding of the tacrine moiety of <scene name='2cmf/Comparison/2'>2d</scene> at the ''Tc''AChE catalytic anionic site (CAS) is similar to that of <font color='red'><b>tacrine </b></font> in the tacrine/''Tc''AChE complex ([[1acj]]). The second tacrine moiety of the <font color='magenta'><b>2d</b></font> interacts with the peripheral anionic site (PAS) near Trp279. The interaction of <font color='magenta'><b>2d</b></font> at the CAS causes an increase of the <scene name='2cmf/Comparison/3'>distance</scene> between Ser200 Oγ and H440 Nε2 atoms, and, therefore, disruption of the catalytic triad (Ser200, H220, E327) as seen in the <font color='cyan'><b>native structure</b></font> ([[2ace]]). The binding of 2d results in <scene name='2cmf/Comparison/4'>major structural changes</scene> in the Val281-Ser291 loop changing the surface of the active-site gorge from its <font color='cyan'><b>native conformation</b></font> ([[2ace]]). The tacrine moiety of the compound <font color='orange'><b>'''2f''' (heptylene-linked bis-tacrine</b></font> at the CAS, [[2ckm]]) adopts similar <scene name='2cmf/Comparison/5'>conformation</scene> as tacrine in the tacrine/''Tc''AChE complex and the tacrine moiety of the <font color='magenta'><b>2d</b></font> at the CAS. The second tacrine moiety of the <font color='orange'><b>2f</b></font> interacts with PAS near the Trp279, like <font color='magenta'><b>2d</b></font>. The <scene name='2cmf/Comparison/6'>binding</scene> of <font color='orange'><b>2f</b></font> does not cause significant structural changes in <font color='plum'><b>''Tc''AChE</b></font> from its <font color='cyan'><b>native structure</b></font>. <scene name='2cmf/Overlap/7'>Comparison</scene> of the structures of <font color='magenta'><b>2d</b></font>/''Tc''AChE and <font color='orange'><b>2f</b></font>/''Tc''AChE reveals different contacts between the tacrine moieties of these compounds at the PAS and ''Tc''AChE. There are two additional structures of tacrine-containing ''Tc''AChE complexes: compounds <scene name='2cmf/Comparison/8'>6</scene> ([[1ut6]]) and <scene name='2cmf/Comparison/9'>7</scene> ([[1odc]]). The tacrine moieties of these compounds adopt similar conformations and interactions with CAS as the tacrine in the tacrine/''Tc''AChE, <font color='orange'><b>2f</b></font>/''Tc''AChE and <font color='magenta'><b>2d</b></font>/''Tc''AChE. Inhibitors '''6''' and '''7''' are spanning the <scene name='2cmf/Comparison/10'>active-site gorge</scene> between the CAS and the PAS, but since compound '''7''' lacks the second tacrine moiety, Trp279 adopts a different conformation in this complex structure. In the three structures: native ''Tc''AChE (cyan), '''cmp 6'''/''Tc''AChE complex (white), and '''cmp 7'''/''Tc''AChE complex (crimson) , all the ''Tc''AChE active-site gorge residues have identical conformation except Trp279 <ref name="Harel">PMID:8415649</ref> <ref name="Raves">PMID:8989325</ref> <ref name="Rydberg">PMID:16942022</ref>. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 10:12, 8 March 2015
| |||||||||||
Additional Resources
For additional information, see: Alzheimer's Disease
References
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- ↑ Botti SA, Felder CE, Lifson S, Sussman JL, Silman I. A modular treatment of molecular traffic through the active site of cholinesterase. Biophys J. 1999 Nov;77(5):2430-50. PMID:10545346
- ↑ 3.0 3.1 3.2 Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat Struct Biol. 1997 Jan;4(1):57-63. PMID:8989325
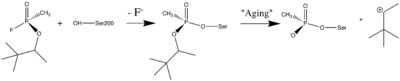
- ↑ Sanson B, Nachon F, Colletier JP, Froment MT, Toker L, Greenblatt HM, Sussman JL, Ashani Y, Masson P, Silman I, Weik M. Crystallographic Snapshots of Nonaged and Aged Conjugates of Soman with Acetylcholinesterase, and of a Ternary Complex of the Aged Conjugate with Pralidoxime (dagger). J Med Chem. 2009 Jul 30. PMID:19642642 doi:10.1021/jm900433t
- ↑ 5.0 5.1 5.2 Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL. Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry. 1999 Jun 1;38(22):7032-9. PMID:10353814 doi:http://dx.doi.org/10.1021/bi982678l
- ↑ 6.0 6.1 Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9031-5. PMID:8415649
- ↑ Dvir H, Wong DM, Harel M, Barril X, Orozco M, Luque FJ, Munoz-Torrero D, Camps P, Rosenberry TL, Silman I, Sussman JL. 3D structure of Torpedo californica acetylcholinesterase complexed with huprine X at 2.1 A resolution: kinetic and molecular dynamic correlates. Biochemistry. 2002 Mar 5;41(9):2970-81. PMID:11863435
- ↑ 8.0 8.1 Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3 A resolution. FEBS Lett. 1999 Dec 17;463(3):321-6. PMID:10606746
- ↑ 9.0 9.1 Ravelli RB, Raves ML, Ren Z, Bourgeois D, Roth M, Kroon J, Silman I, Sussman JL. Static Laue diffraction studies on acetylcholinesterase. Acta Crystallogr D Biol Crystallogr. 1998 Nov 1;54(Pt 6 Pt 2):1359-66. PMID:10089512
- ↑ Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine. Biochemistry. 2002 Mar 19;41(11):3555-64. PMID:11888271
- ↑ Harel M, Sonoda LK, Silman I, Sussman JL, Rosenberry TL. Crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetylcholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site. J Am Chem Soc. 2008 Jun 25;130(25):7856-61. Epub 2008 May 31. PMID:18512913 doi:http://dx.doi.org/10.1021/ja7109822
- ↑ Paz A, Roth E, Ashani Y, Xu Y, Shnyrov VL, Sussman JL, Silman I, Weiner L. Structural and functional characterization of the interaction of the photosensitizing probe methylene blue with Torpedo californica acetylcholinesterase. Protein Sci. 2012 Jun 1. doi: 10.1002/pro.2101. PMID:22674800 doi:10.1002/pro.2101
- ↑ Colletier JP, Bourgeois D, Sanson B, Fournier D, Sussman JL, Silman I, Weik M. Shoot-and-Trap: use of specific x-ray damage to study structural protein dynamics by temperature-controlled cryo-crystallography. Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11742-7. Epub 2008 Aug 13. PMID:18701720
- ↑ 14.0 14.1 Haviv H, Wong DM, Greenblatt HM, Carlier PR, Pang YP, Silman I, Sussman JL. Crystal packing mediates enantioselective ligand recognition at the peripheral site of acetylcholinesterase. J Am Chem Soc. 2005 Aug 10;127(31):11029-36. PMID:16076210 doi:http://dx.doi.org/10.1021/ja051765f
- ↑ Wong DM, Greenblatt HM, Dvir H, Carlier PR, Han YF, Pang YP, Silman I, Sussman JL. Acetylcholinesterase complexed with bivalent ligands related to huperzine a: experimental evidence for species-dependent protein-ligand complementarity. J Am Chem Soc. 2003 Jan 15;125(2):363-73. PMID:12517147 doi:http://dx.doi.org/10.1021/ja021111w
- ↑ Rydberg EH, Brumshtein B, Greenblatt HM, Wong DM, Shaya D, Williams LD, Carlier PR, Pang YP, Silman I, Sussman JL. Complexes of alkylene-linked tacrine dimers with Torpedo californica acetylcholinesterase: Binding of Bis5-tacrine produces a dramatic rearrangement in the active-site gorge. J Med Chem. 2006 Sep 7;49(18):5491-500. PMID:16942022 doi:http://dx.doi.org/10.1021/jm060164b
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, Joel L. Sussman, Michal Harel, Jaime Prilusky, David Canner