| This Sandbox is Reserved from 15/04/2015, through 15/06/2015 for use in the course "Protein structure, function and folding" taught by Taru Meri at the University of Helsinki. This reservation includes Sandbox Reserved 1081 through Sandbox Reserved 1090.
|
To get started:
- Click the edit this page tab at the top. Save the page after each step, then edit it again.
- Click the 3D button (when editing, above the wikitext box) to insert Jmol.
- show the Scene authoring tools, create a molecular scene, and save it. Copy the green link into the page.
- Add a description of your scene. Use the buttons above the wikitext box for bold, italics, links, headlines, etc.
More help: Help:Editing
|
AcrB Transporter
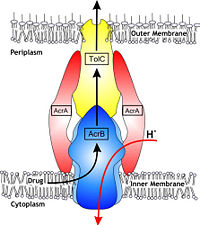
Figure 1. Hypothetical structure of tripartite efflux pumps in Gram-negative bacteria, e.g. the AcrA/AcrB/TolC efflux pump from
E. coli[1]In Gram-negative bacteria, such as Escherichia coli, one of the main reasons behind bacterial multidrug resistance is resistance nodulation cell division (RND) transporters which pump a wide range of dyes, bile salts, detergents and structurally unrelated antibiotics out of the cell by proton motive force driven efflux. The natural function of the efflux system is currently under debate. AcrB is the major RND transporter in E. coli. Three proteins form the tripartite multidrug efflux system that pumps the drugs out from the cell. The AcrB transporter, the inner membrane component of the system, cooperates with two other proteins: membrane fusion protein AcrA and an outer membrane channel TolC. The TolC component is connected to outer membrane and AcrB is connected to the inner membrane. The AcrA connects TolC and AcrB together (Fig 1). The structure of the complex indicates that the drugs are transported out of the cell in a three-step procedure.[1][2][3]
Structure
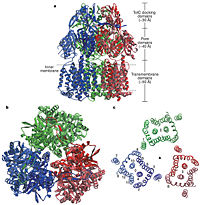
Figure 2. Structure of AcrB. a) Side view of the ribbon representation. The three monomers are individually coloured (blue, green and red), b) Top view of a ribbon representation, c) Structure within a slab of the transmembrane domain parallel to the membrane plane near the periplasmic surface.
[1]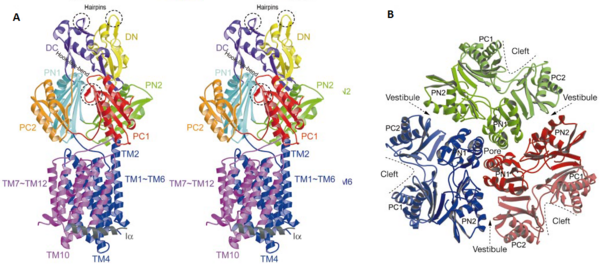
Figure 3. The structure of a single monomer. A) Topology diagram of the protomer, B) A stereo view of ribbon representation of a protomer viewed from outside the pore domain
[1]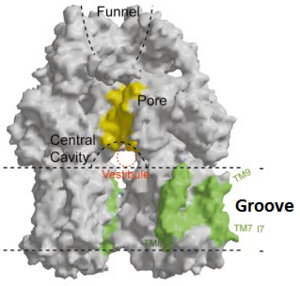
Figure 4. The groove in the transmembrane domain highlighted in green
[1]In the AcrB, there is three domains: TolC docking domain, pore domain and the transmembrane domain. The AcrB consists of 1 049 amino acids and the three monomers of AcrB are organized as a trimer (Fig. 2). The first structure of the trimer was derived in symmetric manner (Fig. 2) at 3.5 Å resolution. Later, a new 2.9 Å asymmetric conformation of trimeric AcrB was obtained and three structurally different monomers were spotted[4]. The appearance of the trimer is of a jellyfish. The N- and C-terminal half’s show similar structural architecture and indicates at early gene duplication events. The trimer, formed by AcrB monomers, appears to be stabilized by the intermonomer connecting loops.[1]
The TolC docking domain, located in the periplasm, has two subdomains: and . The subdomains contain a four-stranded mixed β-sheet. The TolC domain forms a funnel-like structure that has a similar diameter as the bottom of TolC. The TolC docking domain of AcrB and the bottom of TolC fits well with each other for connecting.[1]
The pore domain consists of subdomains , , and (Fig. 3A). These subdomains have a characteristic structural motif: two β-strand–α-helix–β-strand motifs are directly repeated and sandwiched with each other. This motif forms a structure in which two α-helices are located on a four-stranded antiparallel β-sheet. Three α-helices from each PN1 subdomains form a pore in the middle of the structure (Fig. 3B). The pore connects with the bottom of the funnel-like structure of the TolC docking domain. The extramembrane part of the central membrane hole, namely, the central cavity is present at the proximal end of the pore (Fig. 2c). Between PN2 and PC2, there are vestibules open at the side of the pore domain into the periplasm (Fig. 3B). They have access to the central cavity. Analysis with the AcrA has suggested that PC1 and PC2 subdomains are of great importance in attaching the AcrA to the complex. Studies have suggested that C-terminal domain residues play a major role in interacting with AcrA.[1][2]
Twelve α-helices of each monomer forms the transmembrane domain (TM1-12). Six α-helices in the N-terminal and the six in C-terminal are arranged symmetrically. These helices are long and they reach outside the cytoplasmic surface of the membrane. There is an α-helix (Iα) located between TM6 and TM7 in the transmembrane domain. This Iα attaches to the cytoplasmic membrane surface. Between and locates a groove within the transmembrane domain of each monomer. Amino acid residues 860-868 in TM8 are in a disordered state. Through the disordered region of the top of TM8, the groove is connected with the cavity. This domain contains three functionally critical residues: Asp407, Asp408 and Lys940. The residues are shown in this , Asp407 as pink, Asp408 as green and Lys940 as blue. When these are mutated the whole complex loses its drug resistance.[1]
Function
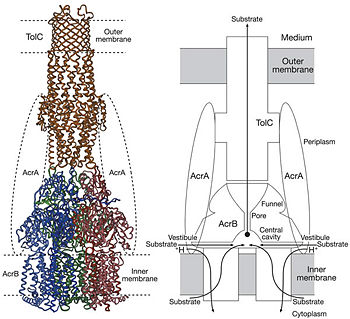
Figure 4. Proposed model of the AcrB/AcrA/TolC complex and the schematic mechanism of multidrug export mediated by AcrAB/TolC system
[1]Possible mechanism of transport function has been postulated. ArcB can cooperate with TolC in the TolC docking domain forming a direct pathway from the cytoplasm to the extracellular milieu. Based on the information of structure, there might be two pathways for the substrate translocation to the central cavity. One is the groove located between TM8 and TM7 in the transmembrane domain of each monoter (Fig 1C). The other is the vestibules which are between PN2 and PC2 of the pore domains. The substrates located in the inner leaflet of the membrane and cytoplasm would get access to the central cavity through the transmembrane groove, while the substrates that are on the outer space or in the outer leaflet of the membrane are more likely to be transported to the cavity through the vestibules.[1]
Studies have been done on the substrate binding and specificity and the periplasmic part of the tripartite efflux system is found important to the substrate specificity. In the study on antibiotics to AcrB, Phe (TM3) was reported as one the main hydrophobic contacts. However, currently, the theoretical explanation of the wide variety of substrates is still lacking.[1]
The tripartite efflux system coupled with proton-motive force across the cytoplasmic membrane. It is the binding and release of protons in the transmembrane domain that is crucial to the energy transduction.[2] Certain key residues had been identified as crucial to the protons translocation. They are the residues Lys940 (TM10) and Asp407 and 408 (TM4) harbored in and in each monomer. When the ion pairs between them are disrupted due to the transient protonation of residues mentioned above, there may be conformational change of TM 4 and TM10. Through possible remote conformational coupling, the conformational change of TM 4 and TM10 may induce the opening of the pore.[1]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Murakami S, Yamaguchi A. [Crystal structure of bacterial multidrug efflux transporter AcrB]. Tanpakushitsu Kakusan Koso. 2003 Jan;48(1):26-32. PMID:12607261
- ↑ 2.0 2.1 2.2 Pos KM. Drug transport mechanism of the AcrB efflux pump. Biochim Biophys Acta. 2009 May;1794(5):782-93. doi: 10.1016/j.bbapap.2008.12.015., Epub 2009 Jan 3. PMID:19166984 doi:http://dx.doi.org/10.1016/j.bbapap.2008.12.015
- ↑ Murakami S. Multidrug efflux transporter, AcrB--the pumping mechanism. Curr Opin Struct Biol. 2008 Aug;18(4):459-65. doi: 10.1016/j.sbi.2008.06.007., Epub 2008 Aug 9. PMID:18644451 doi:http://dx.doi.org/10.1016/j.sbi.2008.06.007
- ↑ Seeger MA, Schiefner A, Eicher T, Verrey F, Diederichs K, Pos KM. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science. 2006 Sep 1;313(5791):1295-8. PMID:16946072 doi:313/5791/1295





