Monoclonal Antibody
From Proteopedia
(Difference between revisions)
| Line 91: | Line 91: | ||
**[[3ls5]] – hFab anti-tetrahydrocannabinol<br /> | **[[3ls5]] – hFab anti-tetrahydrocannabinol<br /> | ||
**[[4g5z]] – hFab canakinumab<br /> | **[[4g5z]] – hFab canakinumab<br /> | ||
| + | **[[1fh5]] – hFab Mak33<br /> | ||
**[[3hmw]] - hFab ustekinumab <br /> | **[[3hmw]] - hFab ustekinumab <br /> | ||
**[[1i9i]] – mFab anti-testosterone<br /> | **[[1i9i]] – mFab anti-testosterone<br /> | ||
| Line 96: | Line 97: | ||
**[[1it9]] - hmFab anti-hFAS<br /> | **[[1it9]] - hmFab anti-hFAS<br /> | ||
**[[1dqm]], [[1dqq]] - mFab anti-lysozyme<br /> | **[[1dqm]], [[1dqq]] - mFab anti-lysozyme<br /> | ||
| + | **[[1g7h]], [[1g7i]], [[1g7j]], [[1g7l]], [[1g7m]] - mFab anti-lysozyme (mutant)<br /> | ||
**[[1ck0]] - mFab anti-angiotensin<br /> | **[[1ck0]] - mFab anti-angiotensin<br /> | ||
**[[2fbj]] – mFab anti-galactan<br /> | **[[2fbj]] – mFab anti-galactan<br /> | ||
| Line 104: | Line 106: | ||
**[[1emt]] – mFab anti-fulerene<br /> | **[[1emt]] – mFab anti-fulerene<br /> | ||
**[[1i3g]] – mFab anti-ampicillin<br /> | **[[1i3g]] – mFab anti-ampicillin<br /> | ||
| - | **[[1j05]] – mFab anti-carcinoembryonic antigen<br /> | + | **[[1j05]], [[1clo]] – mFab anti-carcinoembryonic antigen<br /> |
**[[1jfq]] - mFab anti-phenylarsonate<br /> | **[[1jfq]] - mFab anti-phenylarsonate<br /> | ||
**[[4a6y]] - mFab anti-hydroxy nitrophenyl acetyl<br /> | **[[4a6y]] - mFab anti-hydroxy nitrophenyl acetyl<br /> | ||
| Line 114: | Line 116: | ||
**[[2gsg]] – mFab anti-poly glutamine<br /> | **[[2gsg]] – mFab anti-poly glutamine<br /> | ||
**[[2uyl]] – mFab anti-Vibrio cholera protein<br /> | **[[2uyl]] – mFab anti-Vibrio cholera protein<br /> | ||
| + | **[[1bln]] – mFab anti-glycoprotein<br /> | ||
**[[2v7h]] – mFab anti-mannopyranoside<br /> | **[[2v7h]] – mFab anti-mannopyranoside<br /> | ||
**[[2xkn]] - mFab anti-EGFR<br /> | **[[2xkn]] - mFab anti-EGFR<br /> | ||
| Line 129: | Line 132: | ||
**[[1seq]] - mFab anti-TRKA<br /> | **[[1seq]] - mFab anti-TRKA<br /> | ||
**[[3ojd]] – mFab anti-indolicidin<br /> | **[[3ojd]] – mFab anti-indolicidin<br /> | ||
| - | **[[3gk8]], [[1m71]], [[12e8]], [[1yuh]], [[1fgn]], [[1a6t]], [[4isv]], [[4jj5]], [[3gnm]], [[1ae6]], [[3c5s]], | + | **[[3gk8]], [[1m71]], [[12e8]], [[1yuh]], [[1fgn]], [[1a6t]], [[4isv]], [[4jj5]], [[3gnm]], [[1ae6]], [[3c5s]], [[1mam]], [[3vg0]], [[4kph]], [[1mlb]], [[2vl5]], [[1rmf]], [[2adg]], [[1s5i]], [[1f8t]], [[4dvb]], – mFab <br /> |
**[[3c08]], [[1yy8]] - mFab commercial<br /> | **[[3c08]], [[1yy8]] - mFab commercial<br /> | ||
**[[4ebq]] – mFab anti-vaccinia<br /> | **[[4ebq]] – mFab anti-vaccinia<br /> | ||
| Line 136: | Line 139: | ||
**[[4k7p]] – hFab rhumab6 + mFab 10C4<br /> | **[[4k7p]] – hFab rhumab6 + mFab 10C4<br /> | ||
**[[4idl]] – LgFab anti-cholera toxin<br /> | **[[4idl]] – LgFab anti-cholera toxin<br /> | ||
| + | **[[1c5d]], [[1fn4]] – Fab anti-acetylcholine receptor - rat<br /> | ||
*Anti-HIV Fab | *Anti-HIV Fab | ||
| Line 141: | Line 145: | ||
**[[2pr4]], [[1dfb]], [[3piq]], [[3q6f]], [[3q6g]] - hFab anti-HIV<br /> | **[[2pr4]], [[1dfb]], [[3piq]], [[3q6f]], [[3q6g]] - hFab anti-HIV<br /> | ||
**[[4jo1]], [[4jo2]], [[4jo3]], [[4jo4]] - Fab anti-HIV R56 + gp120 - rabbit<br /> | **[[4jo1]], [[4jo2]], [[4jo3]], [[4jo4]] - Fab anti-HIV R56 + gp120 - rabbit<br /> | ||
| - | **[[3cle]], [[3clf]], [[3cmo]], [[1h3p]], [[3oz9]], [[1mf2]], [[1nld]], [[1bog]], [[3mnv]] - mFab anti-HIV<br /> | + | **[[3cle]], [[3clf]], [[3cmo]], [[1h3p]], [[3oz9]], [[1mf2]], [[1nld]], [[1bog]], [[3mnv]], [[1il1]] - mFab anti-HIV<br /> |
**[[3mo1]] - hmFab anti-gp41<br /> | **[[3mo1]] - hmFab anti-gp41<br /> | ||
| Line 175: | Line 179: | ||
**[[3bae]], [[3bkj]], [[2r0w]], [[2r0z]], [[2ipu]] - mFab + amyloid peptide<br /> | **[[3bae]], [[3bkj]], [[2r0w]], [[2r0z]], [[2ipu]] - mFab + amyloid peptide<br /> | ||
**[[2qhr]] – mFab + Ebola envelope glycoprotein peptide<br /> | **[[2qhr]] – mFab + Ebola envelope glycoprotein peptide<br /> | ||
| + | **[[1ejo]] - mFab + foot-and-mouth virus peptide<br /> | ||
**[[2a6d]], [[2a6i]], [[2a6j]], [[2a6k]] – mFab 36-65 + peptide<br /> | **[[2a6d]], [[2a6i]], [[2a6j]], [[2a6k]] – mFab 36-65 + peptide<br /> | ||
**[[3rkd]] - mFab + HEV-1 capsid peptide<br /> | **[[3rkd]] - mFab + HEV-1 capsid peptide<br /> | ||
| Line 185: | Line 190: | ||
**[[1p4b]] – mFab + GCN4 peptide<br /> | **[[1p4b]] – mFab + GCN4 peptide<br /> | ||
**[[1zea]] – mFab anti-cholera toxin + peptide<br /> | **[[1zea]] – mFab anti-cholera toxin + peptide<br /> | ||
| + | **[[1sm3]] – mFab anti-breast tumor + peptide<br /> | ||
**[[2or9]] - mFab anti-C-myc + peptide<br /> | **[[2or9]] - mFab anti-C-myc + peptide<br /> | ||
**[[4hha]] – hFab anti-cytomegalovirus + glycoprotein B peptide<br /> | **[[4hha]] – hFab anti-cytomegalovirus + glycoprotein B peptide<br /> | ||
| Line 284: | Line 290: | ||
**[[3hpl]], [[2ih1]], [[2ih3]], [[1orq]] - mFab + KcsA K+ channel (mutant) + ion<br /> | **[[3hpl]], [[2ih1]], [[2ih3]], [[1orq]] - mFab + KcsA K+ channel (mutant) + ion<br /> | ||
**[[1ynt]] – mFab 4F11E12 + protein L + major surface antigen p30<br /> | **[[1ynt]] – mFab 4F11E12 + protein L + major surface antigen p30<br /> | ||
| - | **[[2hmi]] – mFab 28 + reverse transcriptase + DNA<br /> | + | **[[2hmi]], [[1n5y]], [[1n6q]], [[1r0a]], [[1hys]] – mFab 28 + reverse transcriptase + DNA<br /> |
**[[3klh]] – mFab + P66 RT + P51 RT + DNA<br /> | **[[3klh]] – mFab + P66 RT + P51 RT + DNA<br /> | ||
**[[3w11]], [[3w12]] , [[3w13]] , [[3w14]] – mFab + insulin + insulin receptor<br /> | **[[3w11]], [[3w12]] , [[3w13]] , [[3w14]] – mFab + insulin + insulin receptor<br /> | ||
| Line 305: | Line 311: | ||
*Fv Fragments | *Fv Fragments | ||
| - | **[[ | + | **[[1ap2]] – mFv C219 <br /> |
| + | **[[1cfv]] – mFv FV4155 <br /> | ||
*Catalytic antibody | *Catalytic antibody | ||
Revision as of 10:28, 5 May 2015
| |||||||||||
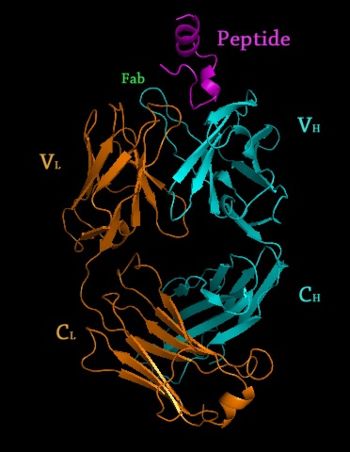
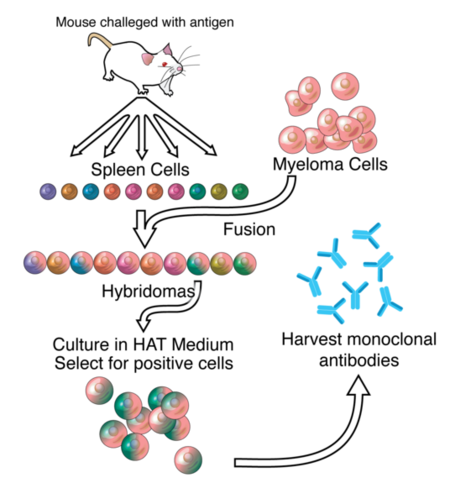
3D structures of Monoclonal Antibodies
Updated on 05-May-2015
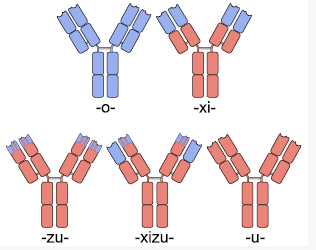
Humanized mouse antibody (hmFab) is a modified mFab which resembles more hFab.
References
- ↑ Roit, I. M. Roit's Essential Immunology. Oxford: Blackwell Science Ltd., 1997.
- ↑ Roit, I. M. Roit's Essential Immunology. Oxford: Blackwell Science Ltd., 1997.
- ↑ Ghosh AK, Spriggs AI, Taylor-Papadimitriou J, Mason DY. Immunocytochemical staining of cells in pleural and peritoneal effusions with a panel of monoclonal antibodies. J Clin Pathol. 1983 Oct;36(10):1154-64. PMID:6194183
- ↑ Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med. 2000 Jun;124(6):921-3. PMID:10835540
- ↑ Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995 Nov;1(11):1311-8. PMID:9815926
- ↑ Brady RL, Edwards DJ, Hubbard RE, Jiang JS, Lange G, Roberts SM, Todd RJ, Adair JR, Emtage JS, King DJ, et al.. Crystal structure of a chimeric Fab' fragment of an antibody binding tumour cells. J Mol Biol. 1992 Sep 5;227(1):253-64. PMID:1522589
- ↑ Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988 Mar 24;332(6162):323-7. PMID:3127726 doi:http://dx.doi.org/10.1038/332323a0
- ↑ Barbas CF 3rd, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7978-82. PMID:1896445
- ↑ Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985 Jun 14;228(4705):1315-7. PMID:4001944
- ↑ Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004 Nov;22(11):1393-8. PMID:15529164 doi:10.1038/nbt1026
- ↑ Choi BK, Bobrowicz P, Davidson RC, Hamilton SR, Kung DH, Li H, Miele RG, Nett JH, Wildt S, Gerngross TU. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5022-7. Epub 2003 Apr 17. PMID:12702754 doi:10.1073/pnas.0931263100
- ↑ van der Kolk LE, Baars JW, Prins MH, van Oers MH. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002 Sep 15;100(6):2257-9. PMID:12200395
- ↑ Jacene HA, Filice R, Kasecamp W, Wahl RL. Comparison of 90Y-ibritumomab tiuxetan and 131I-tositumomab in clinical practice. J Nucl Med. 2007 Nov;48(11):1767-76. Epub 2007 Oct 17. PMID:17942813 doi:10.2967/jnumed.107.043489
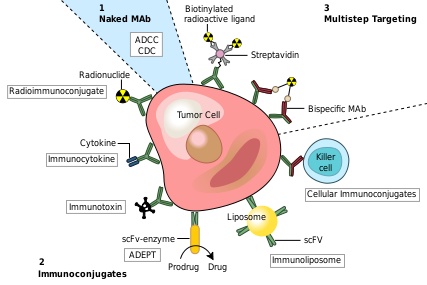
- ↑ Bagshawe KD. Antibody-directed enzyme prodrug therapy (ADEPT) for cancer. Expert Rev Anticancer Ther. 2006 Oct;6(10):1421-31. PMID:17069527 doi:10.1586/14737140.6.10.1421
- ↑ Xu G, McLeod HL. Strategies for enzyme/prodrug cancer therapy. Clin Cancer Res. 2001 Nov;7(11):3314-24. PMID:11705842
- ↑ http://www.nanotech-now.com/news.cgi?story_id=38872
- ↑ Lippow SM, Wittrup KD, Tidor B. Computational design of antibody-affinity improvement beyond in vivo maturation. Nat Biotechnol. 2007 Oct;25(10):1171-6. Epub 2007 Sep 23. PMID:17891135 doi:10.1038/nbt1336
- ↑ Gribenko AV, Patel MM, Liu J, McCallum SA, Wang C, Makhatadze GI. Rational stabilization of enzymes by computational redesign of surface charge-charge interactions. Proc Natl Acad Sci U S A. 2009 Feb 5. PMID:19196981
- ↑ http://f1000scientist.com/article/display/57474/
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky