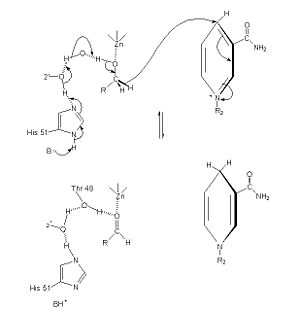
Alcohol dehydrogenase
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
*[[Q165E/S254K Double Mutant Chimera of alcohol dehydrogenase by exchange of the cofactor binding domain res 153-295 of T. brockii ADH by C. beijerinckii ADH]]<br /> | *[[Q165E/S254K Double Mutant Chimera of alcohol dehydrogenase by exchange of the cofactor binding domain res 153-295 of T. brockii ADH by C. beijerinckii ADH]]<br /> | ||
*[[Chimeras of alcohol dehydrogenases]]<br /> | *[[Chimeras of alcohol dehydrogenases]]<br /> | ||
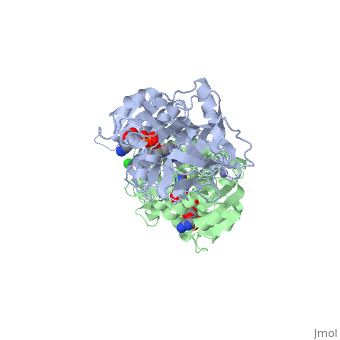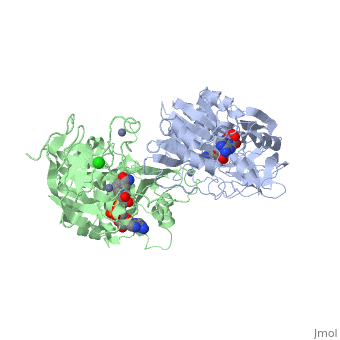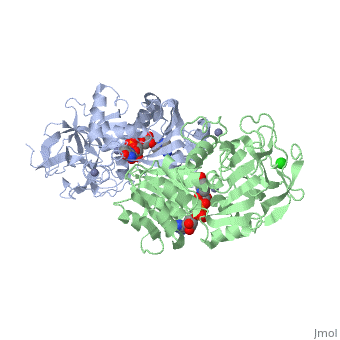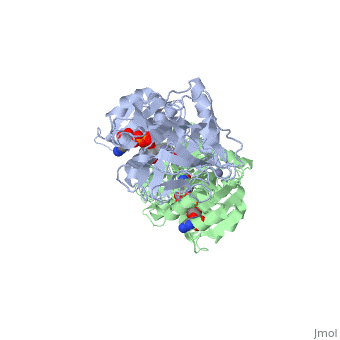
| - | The <scene name='3fsr/Al1/2'>comparison</scene> of overall Cα backbone of all these chimeras (rmsd 0.45-0.65 Å) with those of the parent enzymes, did not reveal significant structural changes. So, the differences in the thermal stability of the chimeras and the parent enzymes could be caused by relatively small specific changes located at the important points of the NADP<sup>+</sup>-dependent alcohol dehydrogenases. For example see Cα superposition for the <font color='red'><b>X23<sub>(TET)</sub> chimera (red)</b></font> ([[3fpc]]) and its parent ADHs (<font color='blue'><b>TbADH, colored blue</b></font> ([[1ped]]), and < | + | The <scene name='3fsr/Al1/2'>comparison</scene> of overall Cα backbone of all these chimeras (rmsd 0.45-0.65 Å) with those of the parent enzymes, did not reveal significant structural changes. So, the differences in the thermal stability of the chimeras and the parent enzymes could be caused by relatively small specific changes located at the important points of the NADP<sup>+</sup>-dependent alcohol dehydrogenases. For example see Cα superposition for the <font color='red'><b>X23<sub>(TET)</sub> chimera (red)</b></font> ([[3fpc]]) and its parent ADHs (<font color='blue'><b>TbADH, colored blue</b></font> ([[1ped]]), and <span style="color:lime;background-color:black;font-weight:bold;">EhADH1, colored green</span> ([[1y9a]]). The [http://en.wikipedia.org/wiki/Root_mean_square_deviation RMSDs] of the TbADH−EhADH1, TbADH−Χ23<sub>(TET)</sub>, and EhADH1−Χ23<sub>(TET)</sub> were 0.68, 0.56, and 0.48 Å, respectively. |
<br/> | <br/> | ||
{{Clear}} | {{Clear}} | ||
| Line 70: | Line 70: | ||
{{Clear}} | {{Clear}} | ||
| - | The <scene name='3fsr/Al1/2'>comparison</scene> of overall Cα backbone of all these chimeras (rmsd 0.45-0.65 Å) with those of the parent enzymes, did not reveal significant structural changes. So, the differences in the thermal stability of the chimeras and the parent enzymes could be caused by relatively small specific changes located at the important points of the NADP<sup>+</sup>-dependent alcohol dehydrogenases. For example see Cα superposition for the <font color='red'><b>X23<sub>(TET)</sub> chimera (red)</b></font> ([[3fpc]]) and its parent ADHs (<font color='blue'><b>TbADH, colored blue</b></font> ([[1ped]]), and < | + | The <scene name='3fsr/Al1/2'>comparison</scene> of overall Cα backbone of all these chimeras (rmsd 0.45-0.65 Å) with those of the parent enzymes, did not reveal significant structural changes. So, the differences in the thermal stability of the chimeras and the parent enzymes could be caused by relatively small specific changes located at the important points of the NADP<sup>+</sup>-dependent alcohol dehydrogenases. For example see Cα superposition for the <font color='red'><b>X23<sub>(TET)</sub> chimera (red)</b></font> ([[3fpc]]) and its parent ADHs (<font color='blue'><b>TbADH, colored blue</b></font> ([[1ped]]), and <span style="color:lime;background-color:black;font-weight:bold;">EhADH1, colored green</span> ([[1y9a]]). The [http://en.wikipedia.org/wiki/Root_mean_square_deviation RMSDs] of the TbADH−EhADH1, TbADH−Χ23<sub>(TET)</sub>, and EhADH1−Χ23<sub>(TET)</sub> were 0.68, 0.56, and 0.48 Å, respectively. |
{{Clear}} | {{Clear}} | ||
| - | The 3D structure of CbADH with the substitution Q100P (<scene name='2b83/Tet/3'>tetramer</scene>) was solved at 2.25 Å resolution ([[2b83]]). The <scene name='2b83/Mut/1'>substitution</scene> of Gln100 with Pro did not cause significant structural changes in the protein structure. The residues of the < | + | The 3D structure of CbADH with the substitution Q100P (<scene name='2b83/Tet/3'>tetramer</scene>) was solved at 2.25 Å resolution ([[2b83]]). The <scene name='2b83/Mut/1'>substitution</scene> of Gln100 with Pro did not cause significant structural changes in the protein structure. The residues of the <span style="color:lime;background-color:black;font-weight:bold;">wildtype protein are colored green</b></font> and the residues of the <font color='cyan'><b>mutant one in cyan</b></font>. Only [http://en.wikipedia.org/wiki/Hydrogen_bond 2 H-bonds] were lost, one between Oε1 of Gln100 and the main chain N of Gly297, and the second between Nε2 of Gln100 and the main chain carbonyl O of Gly297. The mutation caused that an additional CH<sub>2</sub> group (Cδ of Pro100) is surrounded by nonpolar residues: Pro88 (3.8 Å), Trp90 (3.5 Å), and Val95 (4 Å). These residues (P100, P88, W90, and V95) are situated on a protruding lobe of the protein. An additional 11 [http://en.wikipedia.org/wiki/Aliphatic_compound aliphatic] and [http://en.wikipedia.org/wiki/Aromatic aromatic] carbon atoms are situated within the distance of 6 Å from Cδ of Pro100 (two [http://en.wikipedia.org/wiki/Methyl_group methyl groups] of Val95; three carbon atoms of the Trp90 [http://en.wikipedia.org/wiki/Indole indole] group; Cβ and Cγ [http://en.wikipedia.org/wiki/Methylene methylene] groups of Pro100; Cβ and Cγ of Gln101, and two carbons of the Phe99 [http://en.wikipedia.org/wiki/Phenyl_group phenyl] ring). |
{{Clear}} | {{Clear}} | ||
| - | Ribbon diagram of the EhADH1 <scene name='2oui/Tet/1'>tetramer</scene> ([[2oui]]). Proline residues (ball representation) are colored <font color='orange'><b>orange (Pro275)</b></font> (which is important for thermal stabilization) and <font color='cyan'><b>cyan (Pro100)</b></font>. <scene name='2oui/Tet/5'>Superposition</scene> of the structures of the < | + | Ribbon diagram of the EhADH1 <scene name='2oui/Tet/1'>tetramer</scene> ([[2oui]]). Proline residues (ball representation) are colored <font color='orange'><b>orange (Pro275)</b></font> (which is important for thermal stabilization) and <font color='cyan'><b>cyan (Pro100)</b></font>. <scene name='2oui/Tet/5'>Superposition</scene> of the structures of the <span style="color:lime;background-color:black;font-weight:bold;">wild-type apo-EhADH1 (colored green</span>, [[1y9a]]) and the <font color='orange'><b>apo D275P-EhADH1 mutant (colored orange)</b></font> ([[2oui]]). <font color='red'><b>Pro275 and Asp275 are labeled red.</b></font> Residues within a distance of 4 Å from the mutation are shown (names of monomers are in brackets). Replacing <scene name='2oui/Tet/8'>Asp275</scene> with <scene name='2oui/Tet/7'>Pro</scene> significantly enhanced the thermal stability of EhADH1: ΔT<sub>1/2</sub><sup>60min</sup> = +9.3°C, ΔT<sub>1/2</sub><sup>CD</sup> = +10°C. The reverse mutation in the thermophilic <scene name='Tetrameric_alcohol_dehydrogenases/Mut/3'>TbADH</scene> ([[1ykf]]; <font color='magenta'><b>colored magenta</b></font>) - substitution of wt TbADH Pro275 with <scene name='Tetrameric_alcohol_dehydrogenases/Mut/2'>Asp</scene> ([[2nvb]]; <font color='cyan'><b>colored cyan</b></font>) reduced the thermal stability of the enzyme: ΔT<sub>1/2</sub><sup>60min</sup> = -13.8°C, ΔT<sub>1/2</sub><sup>CD</sup> = -18.8°C. Nitrogen and oxygen atoms are colored in [http://en.wikipedia.org/wiki/CPK_coloring CPK colors]. <font color='red'><b>Pro275 and Asp275 are labeled red</b></font> (names of monomers are in brackets). These findings indicate that a single proline mutation is responsible for the significant differences in the thermal stability of ADHs, and show the importance of prolines in the protein stability. It was also shown that substitution by proline at the important positions could significantly stabilize the protein.<ref>PMID 17063493</ref><ref>PMID 18260103</ref><ref>PMID 20102159</ref> |
{{Clear}} | {{Clear}} | ||
Revision as of 13:35, 18 May 2015
| |||||||||||
Additional Resources
For additional information, see: Carbohydrate Metabolism
3D Structures of Alcohol dehydrogenase
Updated on 18-May-2015
References
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol dehydrogenase from Human (Homo sapiens), different isozymes. SCOP. 2009. 1 March 2010 < http://scop.berkeley.edu/data/scop.b.d.c.b.b.c.html>
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Bille V, Remacle J. Simple-kinetic descriptions of alcohol dehydrogenase after immobilization on tresyl-chloride-activated agarose. Eur J Biochem. 1986 Oct 15;160(2):343-8. PMID:3769934
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Blomstrand R, Ostling-Wintzell H, Lof A, McMartin K, Tolf BR, Hedstrom KG. Pyrazoles as inhibitors of alcohol oxidation and as important tools in alcohol research: an approach to therapy against methanol poisoning. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3499-503. PMID:115004
- ↑ Alcohol Dehydrogenase. Worthington Biochemical Corporation . 31 March 2010 < http://http://www.worthington-biochem.com/ADH/default.html>
- ↑ Alcohol Dehydrogenase.Worthington Biochemical Corporation . 31 March 2010 < http://http://www.worthington-biochem.com/ADH/default.html>
- ↑ Goihberg E, Dym O, Tel-Or S, Levin I, Peretz M, Burstein Y. A single proline substitution is critical for the thermostabilization of Clostridium beijerinckii alcohol dehydrogenase. Proteins. 2007 Jan 1;66(1):196-204. PMID:17063493 doi:10.1002/prot.21170
- ↑ Goihberg E, Dym O, Tel-Or S, Shimon L, Frolow F, Peretz M, Burstein Y. Thermal stabilization of the protozoan Entamoeba histolytica alcohol dehydrogenase by a single proline substitution. Proteins. 2008 Feb 7;. PMID:18260103 doi:10.1002/prot.21946
- ↑ Goihberg E, Peretz M, Tel-Or S, Dym O, Shimon L, Frolow F, Burstein Y. Biochemical and Structural Properties of Chimeras Constructed by Exchange of Cofactor-Binding Domains in Alcohol Dehydrogenases from Thermophilic and Mesophilic Microorganisms. Biochemistry. 2010 Feb 9. PMID:20102159 doi:10.1021/bi901730x
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, David Canner, Joel L. Sussman, David Birrer