Chymotrypsin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| + | <StructureSection load='7gch' size='450' side='right' scene='38/387136/Bovine_chymotrypsin_overview/1' caption='[[7gch]] Bovine chymotrypsin with bound inhibitor'> | ||
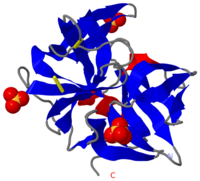
[[Image:2ea3.png|left|200px|thumb|Crystal Structure of ''Cellulomonas Bogoriensis'' Chymotrypsin [[2ea3]]]] | [[Image:2ea3.png|left|200px|thumb|Crystal Structure of ''Cellulomonas Bogoriensis'' Chymotrypsin [[2ea3]]]] | ||
[[Chymotrypsin]] (Chy or α-Chy) is a digestive enzyme containing an active serine residue, which helps to digest proteins in our food. Other related proteases are crucial for blood clotting ([http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=stryer&part=A1378&rendertype=figure&id=A1401 thrombin and other proteases]), for the AIDS virus metabolism ([http://www.proteopedia.org/wiki/index.php/Hiv_protease HIV protease]) and for many other processes relevant to human health and agriculture. Chymotrypsin cleaves peptide bonds of proteins where the amide side of the bond is an aromatic amino acid like tyrosine, phenylalanine or tryptophan. The image at the left is the crystal structure of chymotrypsin from ''Cellulomonas Bogoriensis'' ([[2ea3]]) with sulfate ions. Below is description of the structure of bovine chymotrypsin. Some additional details in<br /> | [[Chymotrypsin]] (Chy or α-Chy) is a digestive enzyme containing an active serine residue, which helps to digest proteins in our food. Other related proteases are crucial for blood clotting ([http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=stryer&part=A1378&rendertype=figure&id=A1401 thrombin and other proteases]), for the AIDS virus metabolism ([http://www.proteopedia.org/wiki/index.php/Hiv_protease HIV protease]) and for many other processes relevant to human health and agriculture. Chymotrypsin cleaves peptide bonds of proteins where the amide side of the bond is an aromatic amino acid like tyrosine, phenylalanine or tryptophan. The image at the left is the crystal structure of chymotrypsin from ''Cellulomonas Bogoriensis'' ([[2ea3]]) with sulfate ions. Below is description of the structure of bovine chymotrypsin. Some additional details in<br /> | ||
| Line 5: | Line 6: | ||
== Overview == | == Overview == | ||
| - | + | While chymotrypsin occurs in many organisms, the most-studied chymotrypsin is that from cows (bovine chymotrypsin), shown here with an inhibitor molecule (shown in [[CPK]]-colored ball and stick) bound to the active site (<scene name='38/387136/Bovine_chymotrypsin_overview/1'>default scene</scene>). It is synthesized as a single polypeptide chain of 245 amino acids, called chymotrypsinogen, which is inactive. The enzyme is activated by one cleavage by trypsin and two cleavages by chymotrypsin (autolytic cleavages) that result in the loss of four amino acids from the remaining three polypeptides, which are shown here in turquoise, beige, and violet. These three chains are held together by <scene name='38/387136/Bovine_chymotrypsin_overview/5'> two inter-chain disulfide bonds</scene>. The bonded cysteine residues are shown in space fill with yellow sulfur atoms. There also three <scene name='38/387136/Bovine_chymotrypsin_overview/6'>intra-chain disulfide bonds</scene>. Here chymotrypsin is shown in cartoon with pink α-helices and yellow β-strands, and this shows that it is mainly composed of <scene name='38/387136/Bovine_chymotrypsin_overview/7'>two beta barrels</scene>. | |
== Substrate-binding and Active Sites == | == Substrate-binding and Active Sites == | ||
Revision as of 12:30, 9 June 2015
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Karsten Theis, Alice Harmon, Alexander Berchansky