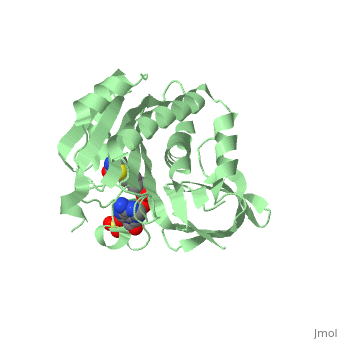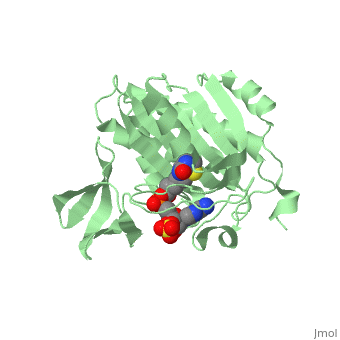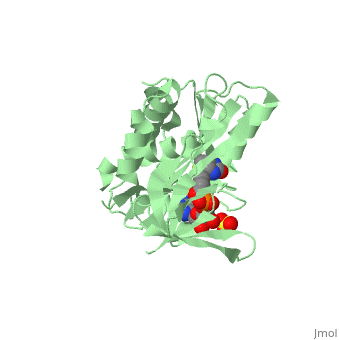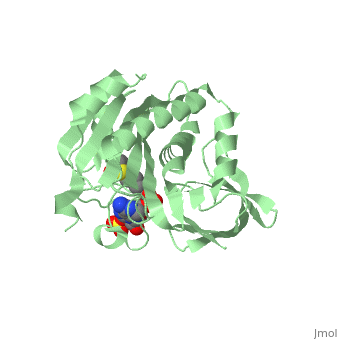Biotin Protein Ligase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='4op0' size='450' side='right' scene= caption='Biotin proteinligase complex with biotinyl-AMP (stick model) and sulfate (PDB code [[4op0]])'> | + | <StructureSection load='4op0' size='450' side='right' scene= caption='Biotin proteinligase complex with biotinyl-5'-AMP (stick model) and sulfate (PDB code [[4op0]])'> |
== Function == | == Function == | ||
| Line 10: | Line 10: | ||
== Structural highlights == | == Structural highlights == | ||
| - | BirA (35.5 kDa) contains three distinct domains that have been determined at 2.3 Å resolution in 1992 through X-ray crystallography in the unliganed form, <scene name='Biotin_Protein_Ligase/Apo_ecbpl/1'>apo_EcBPL</scene> . The monomeric structure measures 75 Å x 35 Å x 30 Å for the unliganded "apo" structure <ref>pmid 1409631</ref>. The <scene name='Biotin_Protein_Ligase/Apo_ecbpl_nterm/3'>N-terminal</scene> 22-46 residues adopt a helix-turn-helix motif, a structure associated with DNA binding proteins. <font color="purple"><scene name='Biotin_Protein_Ligase/Apo_ecbpl_cat/1'>The central domain </scene></font> consists of five α helices, 7 strands of mixed β-sheets as well as four poorly-defined loops that appear in pairs in the 3D structure. These loops consist of residues 110-128, 212-233 and 140 146 and 193-199. The <scene name='Biotin_Protein_Ligase/Apo_ecbpl_cterm/2'>C- terminus</scene> consists of 6 strands which form a β-sandwich that seals the end of the enzyme and has been found to function in the transfer of biotin onto BCCP. | + | BirA (35.5 kDa) contains three distinct domains that have been determined at 2.3 Å resolution in 1992 through X-ray crystallography in the unliganed form, <scene name='Biotin_Protein_Ligase/Apo_ecbpl/1'>apo_EcBPL</scene> . The monomeric structure measures 75 Å x 35 Å x 30 Å for the unliganded "apo" structure <ref>pmid 1409631</ref>. The <scene name='Biotin_Protein_Ligase/Apo_ecbpl_nterm/3'>N-terminal</scene> 22-46 residues adopt a helix-turn-helix motif, a structure associated with DNA binding proteins. <font color="purple"><scene name='Biotin_Protein_Ligase/Apo_ecbpl_cat/1'>The central domain </scene></font> consists of five α helices, 7 strands of mixed β-sheets as well as four poorly-defined loops that appear in pairs in the 3D structure. These loops consist of residues 110-128, 212-233 and 140 146 and 193-199. The <scene name='Biotin_Protein_Ligase/Apo_ecbpl_cterm/2'>C- terminus</scene> consists of 6 strands which form a β-sandwich that seals the end of the enzyme and has been found to function in the transfer of biotin onto BCCP. Upon biotin binding, the protein homodimerises and the unstructured loops become more ordered. The binding pocket of biotinyl-5'-AMP to BirA is shown here. |
| - | Upon biotin binding, the protein homodimerises and the unstructured loops become more ordered. | + | |
==Additional Resources== | ==Additional Resources== | ||
Revision as of 07:40, 12 November 2015
| |||||||||||
3D structures of Biotin Protein Ligase
Updated on 12-November-2015
References
- ↑ Pendini NR, Bailey LM, Booker GW, Wilce MC, Wallace JC, Polyak SW. Microbial biotin protein ligases aid in understanding holocarboxylase synthetase deficiency. Biochim Biophys Acta. 2008 Jul-Aug;1784(7-8):973-82. Epub 2008 Apr 9. PMID:18442489 doi:10.1016/j.bbapap.2008.03.011
- ↑ Bagautdinov B, Kuroishi C, Sugahara M, Kunishima N. Purification, crystallization and preliminary crystallographic analysis of the biotin-protein ligase from Pyrococcus horikoshii OT3. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005 Feb 1;61(Pt, 2):193-5. Epub 2005 Jan 8. PMID:16510991 doi:10.1107/S1744309104034360
- ↑ Wilson KP, Shewchuk LM, Brennan RG, Otsuka AJ, Matthews BW. Escherichia coli biotin holoenzyme synthetase/bio repressor crystal structure delineates the biotin- and DNA-binding domains. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9257-61. PMID:1409631
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Nicole R Pendini, Alexander Berchansky, David Canner, Jaime Prilusky