Leukotriene A4 Hydrolase
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
== Structural highlights == | == Structural highlights == | ||
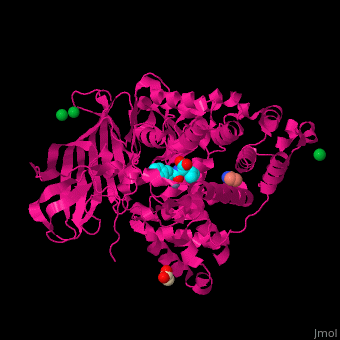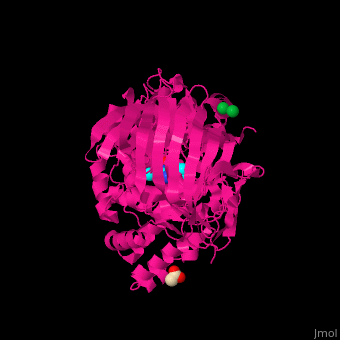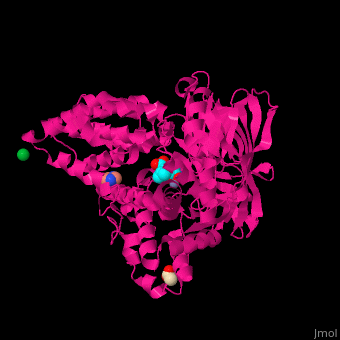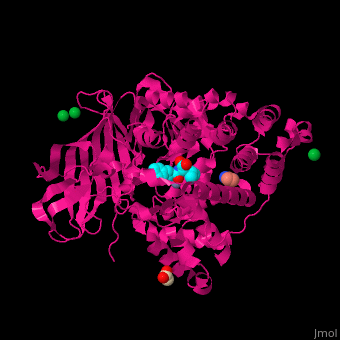
| - | LTA4H structure shows 3 domains: N-terminal, catalytic and C-terminal. The catalytic domain is made of 2 lobes: an α-helical one and an α/β one. The catalytic site is located between the 2 lobes and contains a Zn+2 ion<ref>PMID:11175901</ref> . | + | LTA4H structure shows <scene name='43/433034/Cv/2'>3 domains: N-terminal, catalytic and C-terminal</scene>. The catalytic domain is made of 2 lobes: an α-helical one and an α/β one. The catalytic site is located between the 2 lobes and contains a Zn+2 ion<ref>PMID:11175901</ref> . |
</StructureSection> | </StructureSection> | ||
== 3D Structures of Leukotriene A4 Hydrolase == | == 3D Structures of Leukotriene A4 Hydrolase == | ||
Revision as of 10:42, 17 April 2016
| |||||||||||
3D Structures of Leukotriene A4 Hydrolase
Updated on 17-April-2016
References
- ↑ Paige M, Wang K, Burdick M, Park S, Cha J, Jeffery E, Sherman N, Shim YM. Role of leukotriene A4 hydrolase aminopeptidase in the pathogenesis of emphysema. J Immunol. 2014 Jun 1;192(11):5059-68. doi: 10.4049/jimmunol.1400452. Epub 2014, Apr 25. PMID:24771855 doi:http://dx.doi.org/10.4049/jimmunol.1400452
- ↑ Fourie AM. Modulation of inflammatory disease by inhibitors of leukotriene A4 hydrolase. Curr Opin Investig Drugs. 2009 Nov;10(11):1173-82. PMID:19876785
- ↑ Thunnissen MM, Nordlund P, Haeggstrom JZ. Crystal structure of human leukotriene A(4) hydrolase, a bifunctional enzyme in inflammation. Nat Struct Biol. 2001 Feb;8(2):131-5. PMID:11175901 doi:10.1038/84117