Malate synthase
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Structural highlights == | == Structural highlights == | ||
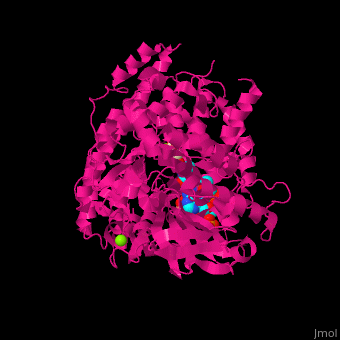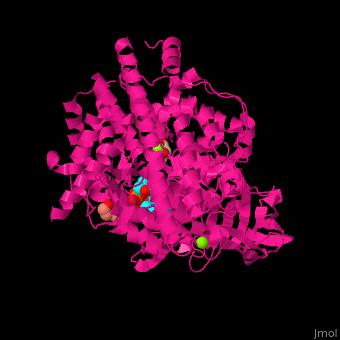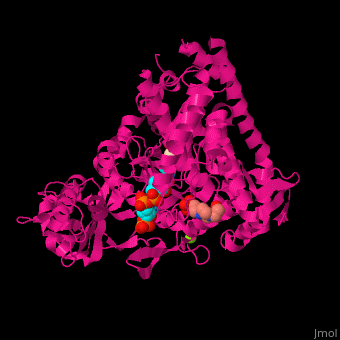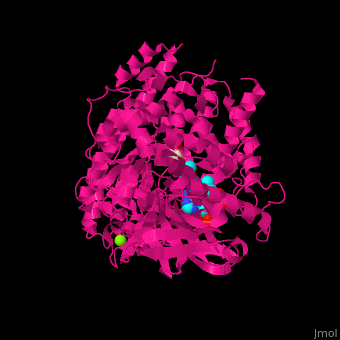
MS active site pocket is situated between the TIM barrle and the C-terminal. The ternary complex contains malate, acetyl-CoA and Mg+2 ion<ref>PMID:16877713</ref>. | MS active site pocket is situated between the TIM barrle and the C-terminal. The ternary complex contains malate, acetyl-CoA and Mg+2 ion<ref>PMID:16877713</ref>. | ||
| + | |||
| + | <scene name='57/573146/Cv/3'>Malate/Mg+2 ion binding site</scene>. Water molecules shown as red spheres. | ||
| + | |||
| + | <scene name='57/573146/Cv/6'>Acetyl-CoA binding site</scene>. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 09:29, 19 April 2016
| |||||||||||
3d structures of malate synthase
Updated on 19-April-2016
References
- ↑ Anstrom DM, Kallio K, Remington SJ. Structure of the Escherichia coli malate synthase G:pyruvate:acetyl-coenzyme A abortive ternary complex at 1.95 A resolution. Protein Sci. 2003 Sep;12(9):1822-32. PMID:12930982
- ↑ Anstrom DM, Remington SJ. The product complex of M. tuberculosis malate synthase revisited. Protein Sci. 2006 Aug;15(8):2002-7. PMID:16877713 doi:15/8/2002
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Karsten Theis, Joel L. Sussman