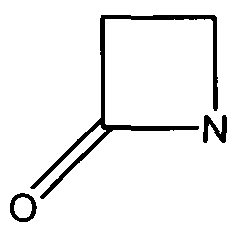
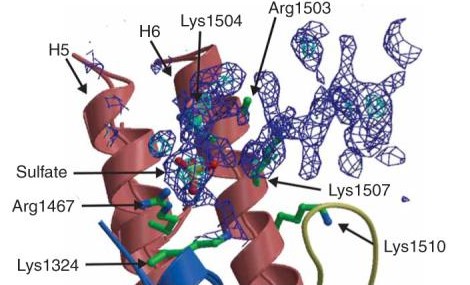
TEM1 Class Antibiotic Resistance Proteins
From Proteopedia
| Line 38: | Line 38: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| - | 1. Davies, J.; Davies, G. Origins and Evolution of Antibiotic Resistance. ''Microbiol Mol Biol Rev.'' | + | 1. Davies, J.; Davies, G. Origins and Evolution of Antibiotic Resistance. (2010) ''Microbiol Mol Biol Rev.'', ''74''(3): 417–433. |
2. National Institute of Health. Stop the Spread of Superbugs Help Fight Drug-Resistant Bacteria. https://newsinhealth.nih.gov/issue/feb2014/feature1. (Last accessed: April 11, 2016). | 2. National Institute of Health. Stop the Spread of Superbugs Help Fight Drug-Resistant Bacteria. https://newsinhealth.nih.gov/issue/feb2014/feature1. (Last accessed: April 11, 2016). | ||
| - | 3. Dablon et al. The catalytic mechanism of f3-lactamases: NMR titration of an active-site lysine residue of the TEM-1 enzyme. ''Proc. Natl. Acad. Sci. USA.'' | + | 3. Dablon et al. (1996) The catalytic mechanism of f3-lactamases: NMR titration of an active-site lysine residue of the TEM-1 enzyme. ''Proc. Natl. Acad. Sci. USA.'' ''74'', 1747-1752. |
| - | 4. Fonze, E.; Charlier, P.; To'th, Y.; Vermeire, M.; Raquet, X.; Dubus, A.; Frere, J. M. TEM1 beta-lactamase structure solved by molecular replacement and refined structure of the S235A mutant. ''Acta Crystallogr. D Biol. Crystallogr.'' | + | 4. Fonze, E.; Charlier, P.; To'th, Y.; Vermeire, M.; Raquet, X.; Dubus, A.; Frere, J. M. (1995) TEM1 beta-lactamase structure solved by molecular replacement and refined structure of the S235A mutant. ''Acta Crystallogr. D Biol. Crystallogr.'' ''51'', 682-694. |
| - | 5. Lenfant, F.; Labia, R.; Masson, J. | + | 5. Lenfant, F.; Labia, R.; Masson, J. (1991) Replacement of lysine 234 affects transition state stabilization in the active site of ß-lactamase TEM1. ''J. Biol. Chem.'' ''266'', 17187-17194. |
Revision as of 22:10, 25 April 2016
| |||||||||||
References
1. Davies, J.; Davies, G. Origins and Evolution of Antibiotic Resistance. (2010) Microbiol Mol Biol Rev., 74(3): 417–433.
2. National Institute of Health. Stop the Spread of Superbugs Help Fight Drug-Resistant Bacteria. https://newsinhealth.nih.gov/issue/feb2014/feature1. (Last accessed: April 11, 2016).
3. Dablon et al. (1996) The catalytic mechanism of f3-lactamases: NMR titration of an active-site lysine residue of the TEM-1 enzyme. Proc. Natl. Acad. Sci. USA. 74, 1747-1752.
4. Fonze, E.; Charlier, P.; To'th, Y.; Vermeire, M.; Raquet, X.; Dubus, A.; Frere, J. M. (1995) TEM1 beta-lactamase structure solved by molecular replacement and refined structure of the S235A mutant. Acta Crystallogr. D Biol. Crystallogr. 51, 682-694.
5. Lenfant, F.; Labia, R.; Masson, J. (1991) Replacement of lysine 234 affects transition state stabilization in the active site of ß-lactamase TEM1. J. Biol. Chem. 266, 17187-17194.
Proteopedia Page Contributors and Editors (what is this?)
Kenna Salvatore, Matt O'Malley, Ryan Hunter Wilson, Riley Culhane, Michal Harel