Structural highlights
1xpb is a 1 chain structure with sequence from Escherichia coli. Full crystallographic information is available from OCA. For a guided tour on the structure components use FirstGlance.
|
| Ligands: | |
| Activity: | Beta-lactamase, with EC number 3.5.2.6 |
| Resources: | FirstGlance, OCA, PDBe, RCSB, PDBsum |
| Related: | 1axb, 1bt5, 1btl, 1ck3, 1erm, 1ero, 1erq, 1esu, 1fqg, 1jtd, 1jtg, 1jvj, 1jwp, 1jwv, 1jwz, 1lhy, 1li0, 1li9, 1m40, 1nxy, 1ny0, 1nym, 1nyy, 1pzo, 1pzp, 1s0w, 1tem, 1xxm, 1yt4, 1zg4, 1zg6, 2v1z |
Background and History
Antibiotics have long been the primary line of defense against many infections. The advent of penicillin in the 1930s brought an end to an era of uncertainty: diseases once considered fatal became treatable. Unfortunately, natural selection stops for no species. Resistance to some form of antibiotic now has become standard for many infections.[A] Moreover, their liberal usage have resulted in “superbugs”, which are resistant to multiple antibiotics.[B] These strains are capable of secreting enzymes capable of deactivating the antibiotic or modifying their cell walls to render the antibiotic ineffective.
Many antibiotics such as penicillin, their derivatives (penams), and cephalosporins (cephams) possess a β-lactam ring (Figure 1).
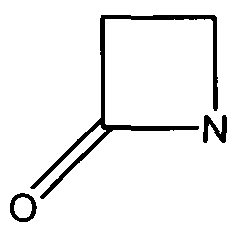
Figure 1. The Beta-Lactam ring is shown above. Beta-lactamases function in hydrolyzing the amide bond within the ring, rendering the antibiotic ineffective. (Image Credit: Wikipedia)
β-Lactamases function by hydrolyzing the β-lactam ring within the antibiotic (Figure 2). This prevents the interaction between the cell wall and the antibiotic.
Figure 2. Bacteria are capable of becoming antibiotic resistant by catalyzing the hydrolysis of the β-lactam ring.
The TEM-1 subclass is one of the most common of all β-Lactamases.[3] Their prevalence began when bacteria started exhibiting penicillin resistance on a mass scale. Since the TEM-1 subclass has been prevalent for many years, many inhibitors have either been discovered or synthesized to prevent their catalytic function.
Function
The β-Lactamases is an enzyme that is a catalyst for hydrolysis. The β-Lactamases have an alpha helix and a beta sheet of five antiparallel strands, which surround the helices. The catalytic region is known as the oxyanion pocket that occurs between the N-terminus of alpha helix H2 and B3 beta sheet edge. Mutations that occur in the catalytic region affect the specificity and catalysis ability of the enzymne which has the ability to slow down the catalysis that inactivates the antibiotics. There are five main steps in the hydrolytic mechanism of TEM-1 β-Lactamases. The mutant enzyme known as AmpC S64G is attached the substrate, then proceeds to an unfavorable high-energy intermediate. this is followed by the formation of an acylated ground state. this is then attacked by water which forms another high-energy intermediate, the rate-determining intermediate. this then forms AmpC and the product.
Disease
Relevance
TEM-1 class antibiotic proteins are probably the most well-known antibiotic resistant protein in the world. Nowadays antibiotic resistance is becoming an ever increasing problem in the medical community. The lives of antibiotics are decreasing as resistant strands of bacteria become more prevalent. In the year 2003, 65% of antibiotics were β-lactamase derivatives. Resistance to these drugs, especially TEM-1, has led to a crisis. Now, over 170 different variants of TEM-1 have been isolated in hospitals across the world. Many of these variants contain different phenotypes making them difficult to combat. TEM-1 is commonly used as a standard in experiments to show how anti-biotic resistant bacteria have evolved over time. This is partly due to the fact of TEM-1 being first documented in the 1960s.
Structural highlights
This TEM1 CLass Antibiotic Resistant protein is similar in structure to other Beta Lactamases in its secondary structure. The protein consists of 236 amino acid residues, which are arranged into a particular structure to provide the function it was designed for. The structure itself consists of two main domains, forming a cleft in between them as the active site for the binding of a sulfate anion ligand. The first domain consists of five beta sheets, with three alpha helices overlaying the side facing the solvent, and another alpha helix flanking the sheets on the adjacent side. The other domain is characterized by its high concentration of alpha helices throughout the secondary structure of this domain. The protein in solution is solvated by 135 water molecules per asymmetric unit. In terms of overall structure, 42.2% consists of alpha helices, 17.5% participate in beta sheets, and 37.2% are turns and coils. Lysine 234, which is present on the wall of the active site as a constituent of one of the beta sheets, has been elucidated as an active binding agent towards substrates, as its amine group is involved in the electrostatic interaction towards penicillins.


