TEM1 Class Antibiotic Resistance Proteins
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
== Background and History == | == Background and History == | ||
| - | Antibiotics have long been the primary line of defense against many infections. The advent of penicillin in the 1930s brought an end to an era of uncertainty: diseases once considered fatal became treatable. Unfortunately, natural selection stops for no species. Resistance to some form of antibiotic now has become the standard for many infections.[ | + | Antibiotics have long been the primary line of defense against many infections. The advent of penicillin in the 1930s brought an end to an era of uncertainty: diseases once considered fatal became treatable. Unfortunately, natural selection stops for no species. Resistance to some form of antibiotic now has become the standard for many infections.[1] Moreover, their liberal usage has resulted in “superbugs”, which are resistant to multiple antibiotics.[2] These strains are capable of secreting enzymes capable of deactivating the antibiotic or modifying their cell walls to render the antibiotic ineffective. |
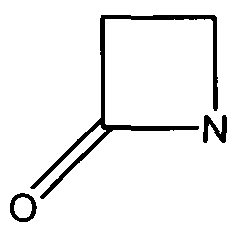
Many antibiotics such as penicillin, their derivatives (penams), and cephalosporins (cephams) possess a β-lactam ring (Figure 1). | Many antibiotics such as penicillin, their derivatives (penams), and cephalosporins (cephams) possess a β-lactam ring (Figure 1). | ||
| Line 31: | Line 31: | ||
== Relevance == | == Relevance == | ||
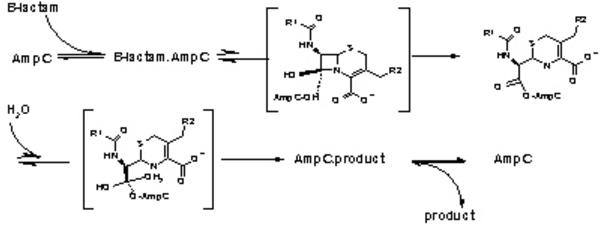
| - | TEM-1 class antibiotic proteins are probably the most well-known antibiotic resistant protein in the world. Nowadays antibiotic resistance is becoming an ever increasing problem in the medical community. The lives of antibiotics are decreasing as resistant strands of bacteria become more prevalent. In the year 2003, 65% of antibiotics were β-lactamase derivatives. Resistance to these drugs, especially TEM-1, has led to a crisis. Now, over 170 different variants of TEM-1 have been isolated in hospitals across the world. Many of these variants contain different phenotypes making them difficult to combat. TEM-1 is commonly used as a standard in experiments to show how antibiotic resistant bacteria have evolved over time. This is partly due to the fact of TEM-1 being first documented in the 1960s. | + | TEM-1 class antibiotic proteins are probably the most well-known antibiotic resistant protein in the world. Nowadays antibiotic resistance is becoming an ever increasing problem in the medical community. The lives of antibiotics are decreasing as resistant strands of bacteria become more prevalent. In the year 2003, 65% of antibiotics were β-lactamase derivatives [5]. Resistance to these drugs, especially TEM-1, has led to a crisis. Now, over 170 different variants of TEM-1 have been isolated in hospitals across the world [6]. Many of these variants contain different phenotypes making them difficult to combat. TEM-1 is commonly used as a standard in experiments to show how antibiotic resistant bacteria have evolved over time. This is partly due to the fact of TEM-1 being first documented in the 1960s [7]. |
== Structural highlights == | == Structural highlights == | ||
| Line 46: | Line 46: | ||
4. Fonze, E.; Charlier, P.; To'th, Y.; Vermeire, M.; Raquet, X.; Dubus, A.; Frere, J. M. (1995) TEM1 beta-lactamase structure solved by molecular replacement and refined structure of the S235A mutant. ''Acta Crystallogr. D Biol. Crystallogr.'' ''51'', 682-694. | 4. Fonze, E.; Charlier, P.; To'th, Y.; Vermeire, M.; Raquet, X.; Dubus, A.; Frere, J. M. (1995) TEM1 beta-lactamase structure solved by molecular replacement and refined structure of the S235A mutant. ''Acta Crystallogr. D Biol. Crystallogr.'' ''51'', 682-694. | ||
| - | 5. | + | 5. Salverda, M. L.; Visser, J. A. G. D.; Barlow, M. Natural Evolution of TEM-1 β-Lactamase: Experimental Reconstruction and Clinical Relevance. FEMS Microbiology Reviews FEMS Microbiol Rev. 2010, 34, 1015–1036. |
| - | 6. Doucet, N.; Savard, P. -.; Pelletier, J. N.; Gagné, S. M. (2007) NMR investigation of Tyr105 mutants in TEM-1 ß-lactamase: Dynamics are correlated with function. ''J. Biol. Chem.'' ''282'', 21448-21459. | + | 6. Neuwirth, C.; Madec, S.; Siebor, E.; Pechinot, A.; Duez, J.-M.; Pruneaux, M.; Fouchereau-Peron, M.; Kazmierczak, A.; Labia, R. TEM-89 Beta -Lactamase Produced by a Proteus Mirabilis Clinical Isolate: New Complex Mutant (CMT 3) with Mutations in Both TEM-59 (IRT-17) and TEM-3. Antimicrobial Agents and Chemotherapy. 2001, 45, 3591–3594. |
| + | |||
| + | 7. Elander, R. P. Industrial Production of β-Lactam Antibiotics. Appl Microbiol Biotechnol Applied Microbiology and Biotechnology. 2003, 61, 385–392. | ||
| + | |||
| + | 8. Lenfant, F.; Labia, R.; Masson, J. (1991) Replacement of lysine 234 affects transition state stabilization in the active site of ß-lactamase TEM1. ''J. Biol. Chem.'' ''266'', 17187-17194. | ||
| + | |||
| + | 9. Doucet, N.; Savard, P. -.; Pelletier, J. N.; Gagné, S. M. (2007) NMR investigation of Tyr105 mutants in TEM-1 ß-lactamase: Dynamics are correlated with function. ''J. Biol. Chem.'' ''282'', 21448-21459. | ||
Revision as of 21:12, 27 April 2016
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Kenna Salvatore, Matt O'Malley, Ryan Hunter Wilson, Riley Culhane, Michal Harel