TEM1 Class Antibiotic Resistance Proteins
From Proteopedia
(Difference between revisions)
| Line 29: | Line 29: | ||
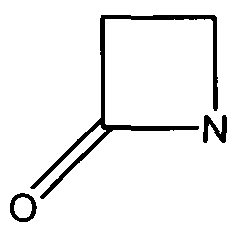
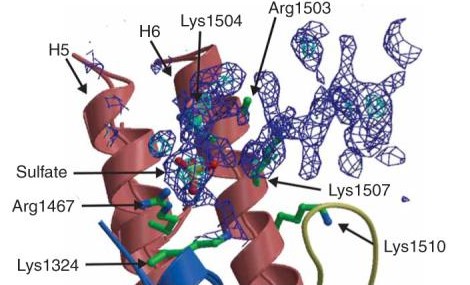
The β-Lactamases has two domains- an alpha helix and a beta sheet of five antiparallel strands, which surround the alpha helix. The catalytic region is known as the oxyanion pocket that occurs between the N-terminus of alpha helix H2 and B3 beta sheet edge. The first step is the acylation of Ser70, one of the catalytic sites. This forms a high-energy acyl-enzyme intermediate, which is then deacylated. This is when the acyl-enzyme is hydrolyzed. The β-Lactam compound has been split and released. The rate determining step is either the acylation or deacylation depending on the antibiotic. However, the opening of the β-Lactam active site increases its chance of inhibition. One of the ways to inhibit β-Lactamases is point mutations in the catalytic region that affect the specificity and catalysis of β-Lactamases enzymes, hindering their activity [4]. | The β-Lactamases has two domains- an alpha helix and a beta sheet of five antiparallel strands, which surround the alpha helix. The catalytic region is known as the oxyanion pocket that occurs between the N-terminus of alpha helix H2 and B3 beta sheet edge. The first step is the acylation of Ser70, one of the catalytic sites. This forms a high-energy acyl-enzyme intermediate, which is then deacylated. This is when the acyl-enzyme is hydrolyzed. The β-Lactam compound has been split and released. The rate determining step is either the acylation or deacylation depending on the antibiotic. However, the opening of the β-Lactam active site increases its chance of inhibition. One of the ways to inhibit β-Lactamases is point mutations in the catalytic region that affect the specificity and catalysis of β-Lactamases enzymes, hindering their activity [4]. | ||
[[Image:Biochem_group_project-function.PNG]] | [[Image:Biochem_group_project-function.PNG]] | ||
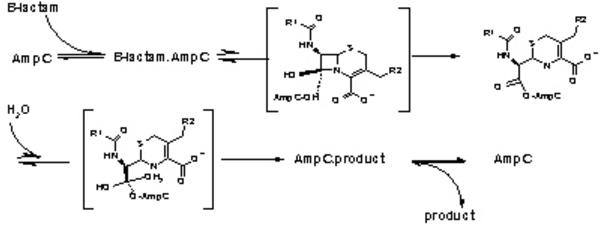
| - | + | Figure 3. The hydrolysis of β-Lactamases where AmpC is the enzyme. There are the three main steps shown with their respective high energy intermediates. (Image Credit: Wikipedia) | |
== Relevance == | == Relevance == | ||
TEM-1 class antibiotic proteins are probably the most well-known antibiotic resistant protein in the world. Nowadays antibiotic resistance is becoming an ever increasing problem in the medical community. The lives of antibiotics are decreasing as resistant strands of bacteria become more prevalent. In the year 2003, 65% of antibiotics were β-lactamase derivatives [5]. Resistance to these drugs, especially TEM-1, has led to a crisis. Now, over 170 different variants of TEM-1 have been isolated in hospitals across the world [6]. Many of these variants contain different phenotypes making them difficult to combat. TEM-1 is commonly used as a standard in experiments to show how antibiotic resistant bacteria have evolved over time. This is partly due to the fact of TEM-1 being first documented in the 1960s [7]. | TEM-1 class antibiotic proteins are probably the most well-known antibiotic resistant protein in the world. Nowadays antibiotic resistance is becoming an ever increasing problem in the medical community. The lives of antibiotics are decreasing as resistant strands of bacteria become more prevalent. In the year 2003, 65% of antibiotics were β-lactamase derivatives [5]. Resistance to these drugs, especially TEM-1, has led to a crisis. Now, over 170 different variants of TEM-1 have been isolated in hospitals across the world [6]. Many of these variants contain different phenotypes making them difficult to combat. TEM-1 is commonly used as a standard in experiments to show how antibiotic resistant bacteria have evolved over time. This is partly due to the fact of TEM-1 being first documented in the 1960s [7]. | ||
Revision as of 21:16, 27 April 2016
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Kenna Salvatore, Matt O'Malley, Ryan Hunter Wilson, Riley Culhane, Michal Harel