TEM1 Class Antibiotic Resistance Proteins
From Proteopedia
(Difference between revisions)
| Line 28: | Line 28: | ||
== Function == | == Function == | ||
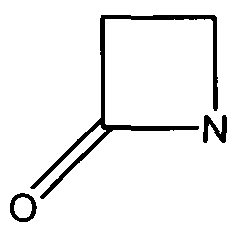
The β-Lactamases has two domains- an alpha helix and a beta sheet of five antiparallel strands, which surround the alpha helix. The catalytic region is known as the oxyanion pocket that occurs between the N-terminus of alpha helix H2 and B3 beta sheet edge. The first step is the acylation of Ser70, one of the catalytic sites. This forms a high-energy acyl-enzyme intermediate, which is then deacylated. This is when the acyl-enzyme is hydrolyzed. The β-Lactam compound has been split and released. The rate determining step is either the acylation or deacylation depending on the antibiotic. However, the opening of the β-Lactam active site increases its chance of inhibition. One of the ways to inhibit β-Lactamases is point mutations in the catalytic region that affect the specificity and catalysis of β-Lactamases enzymes, hindering their activity [4]. | The β-Lactamases has two domains- an alpha helix and a beta sheet of five antiparallel strands, which surround the alpha helix. The catalytic region is known as the oxyanion pocket that occurs between the N-terminus of alpha helix H2 and B3 beta sheet edge. The first step is the acylation of Ser70, one of the catalytic sites. This forms a high-energy acyl-enzyme intermediate, which is then deacylated. This is when the acyl-enzyme is hydrolyzed. The β-Lactam compound has been split and released. The rate determining step is either the acylation or deacylation depending on the antibiotic. However, the opening of the β-Lactam active site increases its chance of inhibition. One of the ways to inhibit β-Lactamases is point mutations in the catalytic region that affect the specificity and catalysis of β-Lactamases enzymes, hindering their activity [4]. | ||
| + | |||
[[Image:Biochem_group_project-function.PNG]] | [[Image:Biochem_group_project-function.PNG]] | ||
| + | |||
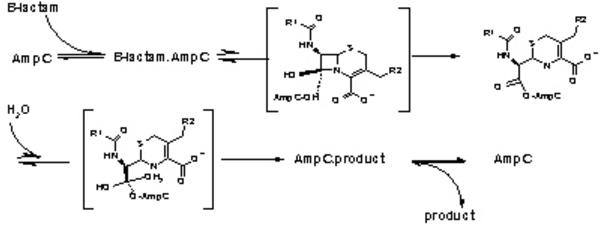
Figure 3. The hydrolysis of β-Lactamases where AmpC is the enzyme. There are the three main steps shown with their respective high energy intermediates. (Image Credit: Shoichet Lab in UCSF) | Figure 3. The hydrolysis of β-Lactamases where AmpC is the enzyme. There are the three main steps shown with their respective high energy intermediates. (Image Credit: Shoichet Lab in UCSF) | ||
== Relevance == | == Relevance == | ||
| Line 46: | Line 48: | ||
4. Fonze, E.; Charlier, P.; To'th, Y.; Vermeire, M.; Raquet, X.; Dubus, A.; Frere, J. M. (1995) TEM1 beta-lactamase structure solved by molecular replacement and refined structure of the S235A mutant. ''Acta Crystallogr. D Biol. Crystallogr.'' ''51'', 682-694. | 4. Fonze, E.; Charlier, P.; To'th, Y.; Vermeire, M.; Raquet, X.; Dubus, A.; Frere, J. M. (1995) TEM1 beta-lactamase structure solved by molecular replacement and refined structure of the S235A mutant. ''Acta Crystallogr. D Biol. Crystallogr.'' ''51'', 682-694. | ||
| - | 5. Salverda, M. L.; Visser, J. A. G. D.; Barlow, M. Natural Evolution of TEM-1 β-Lactamase: Experimental Reconstruction and Clinical Relevance. FEMS Microbiology Reviews FEMS Microbiol Rev. | + | 5. Salverda, M. L.; Visser, J. A. G. D.; Barlow, M. (2010) Natural Evolution of TEM-1 β-Lactamase: Experimental Reconstruction and Clinical Relevance. ''FEMS Microbiology Reviews FEMS Microbiol Rev.'' ''34'', 1015–1036. |
| - | 6. Neuwirth, C.; Madec, S.; Siebor, E.; Pechinot, A.; Duez, J.-M.; Pruneaux, M.; Fouchereau-Peron, M.; Kazmierczak, A.; Labia, R. TEM-89 Beta -Lactamase Produced by a Proteus Mirabilis Clinical Isolate: New Complex Mutant (CMT 3) with Mutations in Both TEM-59 (IRT-17) and TEM-3. Antimicrobial Agents and Chemotherapy | + | 6. Neuwirth, C.; Madec, S.; Siebor, E.; Pechinot, A.; Duez, J.-M.; Pruneaux, M.; Fouchereau-Peron, M.; Kazmierczak, A.; Labia, R. (2010) TEM-89 Beta -Lactamase Produced by a Proteus Mirabilis Clinical Isolate: New Complex Mutant (CMT 3) with Mutations in Both TEM-59 (IRT-17) and TEM-3. ''Antimicrobial Agents and Chemotherapy'' ''45'', 3591–3594. |
| - | 7. Elander, R. P. Industrial Production of β-Lactam Antibiotics. Appl Microbiol Biotechnol Applied Microbiology and Biotechnology. | + | 7. Elander, R. P. (2003) Industrial Production of β-Lactam Antibiotics. ''Appl Microbiol Biotechnol Applied Microbiology and Biotechnology''. ''61'', 385–392. |
8. Lenfant, F.; Labia, R.; Masson, J. (1991) Replacement of lysine 234 affects transition state stabilization in the active site of ß-lactamase TEM1. ''J. Biol. Chem.'' ''266'', 17187-17194. | 8. Lenfant, F.; Labia, R.; Masson, J. (1991) Replacement of lysine 234 affects transition state stabilization in the active site of ß-lactamase TEM1. ''J. Biol. Chem.'' ''266'', 17187-17194. | ||
9. Doucet, N.; Savard, P. -.; Pelletier, J. N.; Gagné, S. M. (2007) NMR investigation of Tyr105 mutants in TEM-1 ß-lactamase: Dynamics are correlated with function. ''J. Biol. Chem.'' ''282'', 21448-21459. | 9. Doucet, N.; Savard, P. -.; Pelletier, J. N.; Gagné, S. M. (2007) NMR investigation of Tyr105 mutants in TEM-1 ß-lactamase: Dynamics are correlated with function. ''J. Biol. Chem.'' ''282'', 21448-21459. | ||
Revision as of 21:26, 27 April 2016
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Kenna Salvatore, Matt O'Malley, Ryan Hunter Wilson, Riley Culhane, Michal Harel