1mwp
From Proteopedia
| Line 7: | Line 7: | ||
|ACTIVITY= | |ACTIVITY= | ||
|GENE= | |GENE= | ||
| + | |DOMAIN= | ||
| + | |RELATEDENTRY= | ||
| + | |RESOURCES=<span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1mwp FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1mwp OCA], [http://www.ebi.ac.uk/pdbsum/1mwp PDBsum], [http://www.rcsb.org/pdb/explore.do?structureId=1mwp RCSB]</span> | ||
}} | }} | ||
| Line 14: | Line 17: | ||
==Overview== | ==Overview== | ||
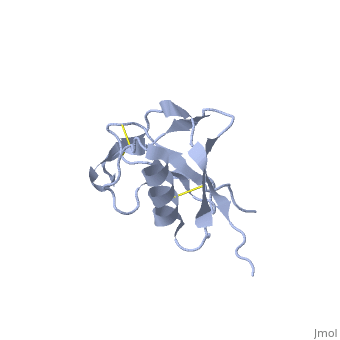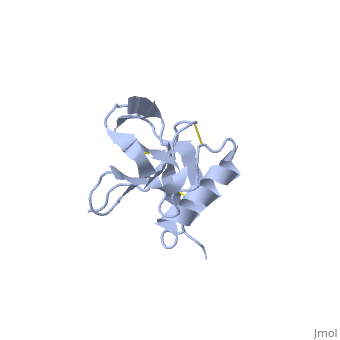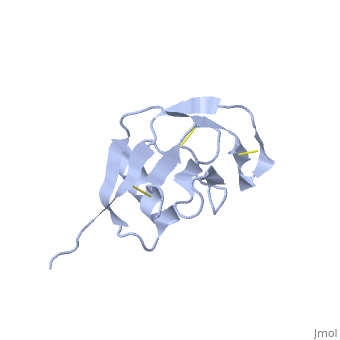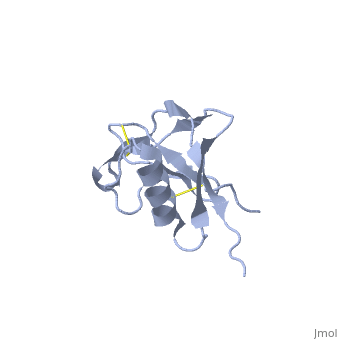
Amyloid precursor protein (APP) plays a central role in Alzheimer disease. A proteolytic-breakdown product of APP, called beta-amyloid, is a major component of the diffuse and fibrillar deposits found in Alzheimer diseased brains. The normal physiological role of APP remains largely unknown despite much work. A knowledge of its function will not only provide insights into the genesis of the disease but may also prove vital in the development of an effective therapy. Here we describe the 1.8 A resolution crystal structure of the N-terminal, heparin-binding domain of APP (residues 28-123), which is responsible, among other things, for stimulation of neurite outgrowth. The structure reveals a highly charged basic surface that may interact with glycosaminoglycans in the brain and an abutting hydrophobic surface that is proposed to play an important functional role such as dimerization or ligand binding. Structural similarities with cysteine-rich growth factors, taken together with its known growth-promoting properties, suggests the APP N-terminal domain could function as a growth factor in vivo. | Amyloid precursor protein (APP) plays a central role in Alzheimer disease. A proteolytic-breakdown product of APP, called beta-amyloid, is a major component of the diffuse and fibrillar deposits found in Alzheimer diseased brains. The normal physiological role of APP remains largely unknown despite much work. A knowledge of its function will not only provide insights into the genesis of the disease but may also prove vital in the development of an effective therapy. Here we describe the 1.8 A resolution crystal structure of the N-terminal, heparin-binding domain of APP (residues 28-123), which is responsible, among other things, for stimulation of neurite outgrowth. The structure reveals a highly charged basic surface that may interact with glycosaminoglycans in the brain and an abutting hydrophobic surface that is proposed to play an important functional role such as dimerization or ligand binding. Structural similarities with cysteine-rich growth factors, taken together with its known growth-promoting properties, suggests the APP N-terminal domain could function as a growth factor in vivo. | ||
| - | |||
| - | ==Disease== | ||
| - | Known diseases associated with this structure: Alzheimer disease-1, APP-related OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=104760 104760]], Amyloidosis, cerebroarterial, Dutch type OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=104760 104760]], Amyloidosis, cerebroarterial, Iowa type OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=104760 104760]], Blood group, P system OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=607922 607922]] | ||
==About this Structure== | ==About this Structure== | ||
| Line 39: | Line 39: | ||
[[Category: heparin binding]] | [[Category: heparin binding]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Sun Mar 30 22:22:10 2008'' |
Revision as of 19:22, 30 March 2008
| |||||||
| , resolution 1.8Å | |||||||
|---|---|---|---|---|---|---|---|
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
N-TERMINAL DOMAIN OF THE AMYLOID PRECURSOR PROTEIN
Overview
Amyloid precursor protein (APP) plays a central role in Alzheimer disease. A proteolytic-breakdown product of APP, called beta-amyloid, is a major component of the diffuse and fibrillar deposits found in Alzheimer diseased brains. The normal physiological role of APP remains largely unknown despite much work. A knowledge of its function will not only provide insights into the genesis of the disease but may also prove vital in the development of an effective therapy. Here we describe the 1.8 A resolution crystal structure of the N-terminal, heparin-binding domain of APP (residues 28-123), which is responsible, among other things, for stimulation of neurite outgrowth. The structure reveals a highly charged basic surface that may interact with glycosaminoglycans in the brain and an abutting hydrophobic surface that is proposed to play an important functional role such as dimerization or ligand binding. Structural similarities with cysteine-rich growth factors, taken together with its known growth-promoting properties, suggests the APP N-terminal domain could function as a growth factor in vivo.
About this Structure
1MWP is a Single protein structure of sequence from Homo sapiens. The following page contains interesting information on the relation of 1MWP with [Amyloid-beta Precursor Protein]. Full crystallographic information is available from OCA.
Reference
Crystal structure of the N-terminal, growth factor-like domain of Alzheimer amyloid precursor protein., Rossjohn J, Cappai R, Feil SC, Henry A, McKinstry WJ, Galatis D, Hesse L, Multhaup G, Beyreuther K, Masters CL, Parker MW, Nat Struct Biol. 1999 Apr;6(4):327-31. PMID:10201399
Page seeded by OCA on Sun Mar 30 22:22:10 2008