We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Elongation factor
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
'''Elongation factors''' (EF) facilitate translational elongation during the formation of peptide bonds in the ribosome.<br /> | '''Elongation factors''' (EF) facilitate translational elongation during the formation of peptide bonds in the ribosome.<br /> | ||
| - | * '''EF-selB''' is selenocysteine-specific EF.<br /> | + | * '''EF-selB''' is selenocysteine-specific EF. See [[SelB]]<br /> |
* '''EF-Tu or EF 1-α''' (elongation factor thermo unstable) is a prokaryotic EF. EF-Tu contributes to translational accuracy. It catalyzes the addition of aminoacyl tRNA<ref>PMID:20798060</ref>. <br /> | * '''EF-Tu or EF 1-α''' (elongation factor thermo unstable) is a prokaryotic EF. EF-Tu contributes to translational accuracy. It catalyzes the addition of aminoacyl tRNA<ref>PMID:20798060</ref>. <br /> | ||
*'''EF-Ts or EF 1-β''' (elongation factor thermo stable) catalyzes the release of GDP from EF-Tu.<br /> | *'''EF-Ts or EF 1-β''' (elongation factor thermo stable) catalyzes the release of GDP from EF-Tu.<br /> | ||
| Line 13: | Line 13: | ||
* '''EF-1 γ''' acts during the delivery of aminoacyl tRNA to the ribosome.<br /> | * '''EF-1 γ''' acts during the delivery of aminoacyl tRNA to the ribosome.<br /> | ||
* '''EF-2''' promotes the translocation of the nascent protein chain from the A site to the P site on the ribosome<ref>PMID:16246167</ref>.<br /> | * '''EF-2''' promotes the translocation of the nascent protein chain from the A site to the P site on the ribosome<ref>PMID:16246167</ref>.<br /> | ||
| - | * '''EF-3''' is a unique EF in fungi hence it provides an anti-fungal drug target.<br /> | + | * '''EF-3''' is a unique EF in fungi hence it provides an anti-fungal drug target. See [[HEAT Repeat]]<br /> |
* '''EF Spt4, Spt5, Spt6''' are conserved among eukaryotes. They modulate the chromatin structure.<br /> | * '''EF Spt4, Spt5, Spt6''' are conserved among eukaryotes. They modulate the chromatin structure.<br /> | ||
* '''EF-CA150''' is believed to play a role in coupling transcription and splicing.<br /> | * '''EF-CA150''' is believed to play a role in coupling transcription and splicing.<br /> | ||
| Line 19: | Line 19: | ||
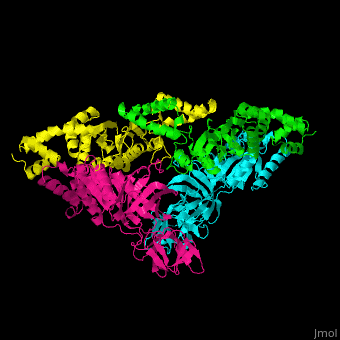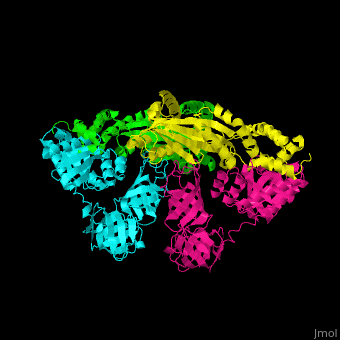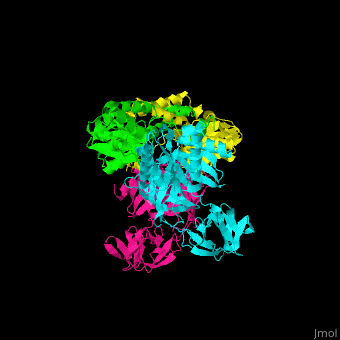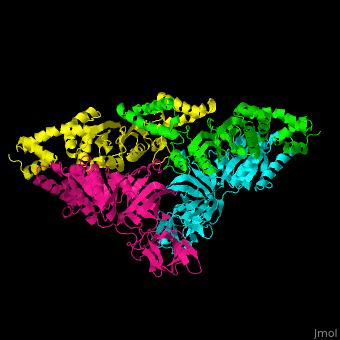
<scene name='51/517376/Cv/2'>Complex EF-Tu with EF-Ts is heterotetramer</scene>, or, more exactly <scene name='51/517376/Cv/3'>heterodimer of homodimers</scene> (PDB entry [[1efu]]).<ref>PMID:8596629</ref> | <scene name='51/517376/Cv/2'>Complex EF-Tu with EF-Ts is heterotetramer</scene>, or, more exactly <scene name='51/517376/Cv/3'>heterodimer of homodimers</scene> (PDB entry [[1efu]]).<ref>PMID:8596629</ref> | ||
| - | |||
| - | For EF-3 see [[HEAT Repeat]]<br /> | ||
| - | For EF-SelB see [[SelB]]. | ||
</StructureSection> | </StructureSection> | ||
| Line 203: | Line 200: | ||
**[[1wsu]], [[2uwm]] – MtEF + RNA<BR /> | **[[1wsu]], [[2uwm]] – MtEF + RNA<BR /> | ||
**[[2ply]] – MtEF (mutant) + RNA<BR /> | **[[2ply]] – MtEF (mutant) + RNA<BR /> | ||
| - | ** | + | **[[4aca]] – MmEF – ''Methanococcus maripaludis''<BR /> |
| - | ** | + | **[[4acb]] – MmEF + GTP analog<br /> |
| - | **[[4ac9 | + | **[[4ac9]] – MmEF + GDP<BR /> |
**[[2pjp]] – EcEF + RNA | **[[2pjp]] – EcEF + RNA | ||
Revision as of 11:41, 16 November 2016
| |||||||||||
3D structures of elongation factor
Updated on 16-November-2016
References
- ↑ Takeshita D, Tomita K. Assembly of Q{beta} viral RNA polymerase with host translational elongation factors EF-Tu and -Ts. Proc Natl Acad Sci U S A. 2010 Sep 7;107(36):15733-8. Epub 2010 Aug 23. PMID:20798060 doi:http://dx.doi.org/10.1073/pnas.1006559107
- ↑ Jorgensen R, Merrill AR, Andersen GR. The life and death of translation elongation factor 2. Biochem Soc Trans. 2006 Feb;34(Pt 1):1-6. PMID:16246167 doi:http://dx.doi.org/10.1042/BST20060001
- ↑ Kawashima T, Berthet-Colominas C, Wulff M, Cusack S, Leberman R. The structure of the Escherichia coli EF-Tu.EF-Ts complex at 2.5 A resolution. Nature. 1996 Feb 8;379(6565):511-8. PMID:8596629 doi:http://dx.doi.org/10.1038/379511a0