User:Camille Zumstein/Sandbox
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='4il1' size='340' side='right' caption='Rat calcineurin monomer' scene=''> | <StructureSection load='4il1' size='340' side='right' caption='Rat calcineurin monomer' scene=''> | ||
| + | <StructureSection load='4il1' size='340' side='right' caption='Rat calcineurin monomer' scene='<scene name='75/750223/Picture_intro/1'>cqln</scene>'> | ||
| + | |||
| + | |||
Calcineurin is a serine/threonine phosphatase that is activated by [https://en.wikipedia.org/wiki/Calmodulin calmodulin] in a calcium-dependant manner. Calcineurin is the only known calmodulin-activated protein phosphatase. Calcineurin is responsible mainly for dephosphorylating several member of the [https://en.wikipedia.org/wiki/NFAT NFAT] transcription factor family (including NFATc1 through NFATc4). | Calcineurin is a serine/threonine phosphatase that is activated by [https://en.wikipedia.org/wiki/Calmodulin calmodulin] in a calcium-dependant manner. Calcineurin is the only known calmodulin-activated protein phosphatase. Calcineurin is responsible mainly for dephosphorylating several member of the [https://en.wikipedia.org/wiki/NFAT NFAT] transcription factor family (including NFATc1 through NFATc4). | ||
''' Localisation and expression''': | ''' Localisation and expression''': | ||
Revision as of 09:58, 10 January 2017
Structure Rat Calcineurin
| |||||||||||
References
- ↑ http://www.jimmunol.org/content/177/4/2681.full
- ↑ http://www.uniprot.org/uniprot/P63329
- ↑ http://www.uniprot.org/uniprot/P63329
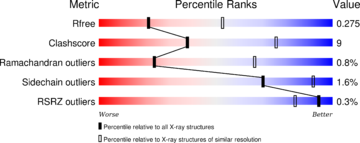
- ↑ Ye Q, Feng Y, Yin Y, Faucher F, Currie MA, Rahman MN, Jin J, Li S, Wei Q, Jia Z. Structural basis of calcineurin activation by calmodulin. Cell Signal. 2013 Sep 7;25(12):2661-2667. doi: 10.1016/j.cellsig.2013.08.033. PMID:24018048 doi:10.1016/j.cellsig.2013.08.033
- ↑ http://www.rcsb.org/pdb/explore/explore.do?structureId=4IL1
- ↑ Rumi-Masante J, Rusinga FI, Lester TE, Dunlap TB, Williams TD, Dunker AK, Weis DD, Creamer TP. Structural basis for activation of calcineurin by calmodulin. J Mol Biol. 2012 Jan 13;415(2):307-17. doi: 10.1016/j.jmb.2011.11.008. Epub 2011 , Nov 12. PMID:22100452 doi:http://dx.doi.org/10.1016/j.jmb.2011.11.008
- ↑ Takeuchi K, Roehrl MH, Sun ZY, Wagner G. Structure of the calcineurin-NFAT complex: defining a T cell activation switch using solution NMR and crystal coordinates. Structure. 2007 May;15(5):587-97. PMID:17502104 doi:10.1016/j.str.2007.03.015
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/22654726
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/8811062
- ↑ http://www.uptodate.com/contents/pharmacology-of-cyclosporine-and-tacrolimus
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/8837775
- ↑ Calmodulin and Signal Transduction (p184), Linda J. Van Eldik,D. Martin Watterson (1998)
- ↑ http://www.uptodate.com/contents/pharmacology-of-cyclosporine-and-tacrolimus
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/12851457
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/16988714
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2857609/
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/8837775