User:Camille Zumstein/Sandbox
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
== General structure == | == General structure == | ||
| - | <scene name='75/750223/Jw_caln_sec_strcutur/1'>Calcineurin</scene> is a heterodimeric Protein that consits of two subunits. They are called catalytic and regulatory subunit. Four molecules of Calcineurin form | + | <scene name='75/750223/Jw_caln_sec_strcutur/1'>Calcineurin</scene> is a heterodimeric Protein that consits of two subunits. They are called catalytic and regulatory subunit. Four molecules of Calcineurin form an asymmetric complex which leads to a total molecular weight of 370 kDa. |
| - | The structure presented in this article is the catalytic subunit isoform of the serine/threonine-protein phosphatase 2B in rattus norvegicus (rat). It consists of 521 <ref>http://www.uniprot.org/uniprot/P63329</ref> aminoacids and has a molecular weight of 57 kDa <ref>http://www.uniprot.org/uniprot/P63329</ref>. | + | The structure presented in this article is the catalytic subunit isoform of the serine/threonine-protein phosphatase 2B in [https://www.ncbi.nlm.nih.gov/UniGene/UGOrg.cgi?TAXID=10116 rattus norvegicus (rat)]. It consists of 521 <ref>http://www.uniprot.org/uniprot/P63329</ref> aminoacids and has a molecular weight of 57 kDa <ref>http://www.uniprot.org/uniprot/P63329</ref>. |
The calatytic subunit is subdivided into functional domains which are a <scene name='75/750223/Catalytique_domain_of_chain_a/1'>catalytic domain (here chain A is shown)</scene>, a <scene name='75/750223/Interact_dom_ca/1'>binding domain for the regulary subunit</scene>, a <scene name='75/750223/Calm_bind_dom_ca/1'>calmodulin binding domain </scene> and an <scene name='75/750223/Auto_inh_dom_ca/1'>autoinhibitory domain</scene>. | The calatytic subunit is subdivided into functional domains which are a <scene name='75/750223/Catalytique_domain_of_chain_a/1'>catalytic domain (here chain A is shown)</scene>, a <scene name='75/750223/Interact_dom_ca/1'>binding domain for the regulary subunit</scene>, a <scene name='75/750223/Calm_bind_dom_ca/1'>calmodulin binding domain </scene> and an <scene name='75/750223/Auto_inh_dom_ca/1'>autoinhibitory domain</scene>. | ||
| Line 24: | Line 24: | ||
'''Discovery of the structure:''' | '''Discovery of the structure:''' | ||
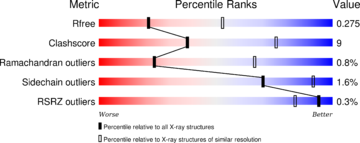
[[Image:4il1 multipercentile validation.png|thumb|upright=2|left]] | [[Image:4il1 multipercentile validation.png|thumb|upright=2|left]] | ||
| - | The structure have been published in the year 2013 by Qilu Ye et al. <ref>PMID:24018048</ref>. For their experiments they used [https://en.wikipedia.org/wiki/X-ray_crystallography#X-ray_diffraction x-ray diffraction]. The PDB validation obtained a Resolutionof 3.0 Å, a free R-value of 0.273 and a work R-value of 0.241 <ref>http://www.rcsb.org/pdb/explore/explore.do?structureId=4IL1</ref>. | + | The structure have been [https://www.ncbi.nlm.nih.gov/pubmed/24018048 published in the year 2013 by Qilu Ye et al.] <ref>PMID:24018048</ref> in [http://www.sciencedirect.com/science/journal/08986568 ''Cellular Signaling'']. For their experiments they used [https://en.wikipedia.org/wiki/X-ray_crystallography#X-ray_diffraction x-ray diffraction]. The [http://www.wwpdb.org/validation/2016/XrayValidationReportHelp PDB validation] obtained a Resolutionof 3.0 Å, a free R-value of 0.273 and a work R-value of 0.241 <ref>http://www.rcsb.org/pdb/explore/explore.do?structureId=4IL1</ref>. |
<br style="clear:both" /> | <br style="clear:both" /> | ||
| Line 32: | Line 32: | ||
[[Image:170106 Uniprot SecundaryStructure.png|thumb|upright=4|The secondary structure mostly includes helical structures]] | [[Image:170106 Uniprot SecundaryStructure.png|thumb|upright=4|The secondary structure mostly includes helical structures]] | ||
| - | Besides, there are six turns reported in the structure of | + | Besides, there are six turns reported in the structure of one chain. |
<br style="clear:both" /> | <br style="clear:both" /> | ||
Revision as of 08:11, 14 January 2017
Structure Rat Calcineurin
| |||||||||||
References
- ↑ Yoo SA, Park BH, Park GS, Koh HS, Lee MS, Ryu SH, Miyazawa K, Park SH, Cho CS, Kim WU. Calcineurin is expressed and plays a critical role in inflammatory arthritis. J Immunol. 2006 Aug 15;177(4):2681-90. PMID:16888030
- ↑ http://www.uniprot.org/uniprot/P63329
- ↑ http://www.uniprot.org/uniprot/P63329
- ↑ Ye Q, Feng Y, Yin Y, Faucher F, Currie MA, Rahman MN, Jin J, Li S, Wei Q, Jia Z. Structural basis of calcineurin activation by calmodulin. Cell Signal. 2013 Sep 7;25(12):2661-2667. doi: 10.1016/j.cellsig.2013.08.033. PMID:24018048 doi:10.1016/j.cellsig.2013.08.033
- ↑ http://www.rcsb.org/pdb/explore/explore.do?structureId=4IL1
- ↑ Rumi-Masante J, Rusinga FI, Lester TE, Dunlap TB, Williams TD, Dunker AK, Weis DD, Creamer TP. Structural basis for activation of calcineurin by calmodulin. J Mol Biol. 2012 Jan 13;415(2):307-17. doi: 10.1016/j.jmb.2011.11.008. Epub 2011 , Nov 12. PMID:22100452 doi:http://dx.doi.org/10.1016/j.jmb.2011.11.008
- ↑ Takeuchi K, Roehrl MH, Sun ZY, Wagner G. Structure of the calcineurin-NFAT complex: defining a T cell activation switch using solution NMR and crystal coordinates. Structure. 2007 May;15(5):587-97. PMID:17502104 doi:10.1016/j.str.2007.03.015
- ↑ Kingsbury TJ, Bambrick LL, Roby CD, Krueger BK. Calcineurin activity is required for depolarization-induced, CREB-dependent gene transcription in cortical neurons. J Neurochem. 2007 Oct;103(2):761-70. Epub 2007 Jul 31. PMID:17666045 doi:http://dx.doi.org/10.1111/j.1471-4159.2007.04801.x
- ↑ Karch CM, Jeng AT, Goate AM. Calcium phosphatase calcineurin influences tau metabolism. Neurobiol Aging. 2013 Feb;34(2):374-86. doi:, 10.1016/j.neurobiolaging.2012.05.003. Epub 2012 Jun 6. PMID:22676853 doi:http://dx.doi.org/10.1016/j.neurobiolaging.2012.05.003
- ↑ Hilioti Z, Gallagher DA, Low-Nam ST, Ramaswamy P, Gajer P, Kingsbury TJ, Birchwood CJ, Levchenko A, Cunningham KW. GSK-3 kinases enhance calcineurin signaling by phosphorylation of RCNs. Genes Dev. 2004 Jan 1;18(1):35-47. Epub 2003 Dec 30. PMID:14701880 doi:http://dx.doi.org/10.1101/gad.1159204
- ↑ Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994 Jun 9;369(6480):486-8. PMID:7515479 doi:http://dx.doi.org/10.1038/369486a0
- ↑ Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996 Sep;80(3 Pt 2):S40-5. PMID:8811062
- ↑ http://www.uptodate.com/contents/pharmacology-of-cyclosporine-and-tacrolimus
- ↑ Wang X, Culotta VC, Klee CB. Superoxide dismutase protects calcineurin from inactivation. Nature. 1996 Oct 3;383(6599):434-7. PMID:8837775 doi:http://dx.doi.org/10.1038/383434a0
- ↑ Calmodulin and Signal Transduction (p184), Linda J. Van Eldik,D. Martin Watterson (1998)
- ↑ http://www.uptodate.com/contents/pharmacology-of-cyclosporine-and-tacrolimus
- ↑ Miyakawa T, Leiter LM, Gerber DJ, Gainetdinov RR, Sotnikova TD, Zeng H, Caron MG, Tonegawa S. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):8987-92. Epub 2003 Jul 8. PMID:12851457 doi:http://dx.doi.org/10.1073/pnas.1432926100
- ↑ Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006 Sep 21;443(7109):345-9. PMID:16988714 doi:http://dx.doi.org/10.1038/nature05097
- ↑ Lampropoulos CE, D'Cruz DP. Topical calcineurin inhibitors in systemic lupus erythematosus. Ther Clin Risk Manag. 2010 Apr 15;6:95-101. PMID:20421909
- ↑ Reese LC, Taglialatela G. A role for calcineurin in Alzheimer's disease. Curr Neuropharmacol. 2011 Dec;9(4):685-92. doi: 10.2174/157015911798376316. PMID:22654726 doi:http://dx.doi.org/10.2174/157015911798376316