|
|
| (155 intermediate revisions not shown.) |
| Line 1: |
Line 1: |
| - | ==Your Heading Here (maybe something like 'Structure')== 2
| + | '''Grb2 (1gri)''' |
| - | <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | + | <StructureSection load='1gri' size='340' side='right' caption='Caption for this structure' scene=''> |
| - | This is a default text for your page '''Charli Barbet/Sandbox'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs.
| + | '''Growth Factor Receptor Bound Protein (Grb2)''' is a cytosolic protein made of 217 amino acids and weighing 25,206 Da. Ubiquitously present in the cell, the protein is involved in signal transduction and plays a major role in the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MAP kinase pathway]. Grb2 interacts mainly with [https://en.wikipedia.org/wiki/Tyrosine_kinase '''tyrosine kinases'''] such as [http://www.uniprot.org/uniprot/P00533 EGFR]. When EGFR is activated by ligand binding, a Guanine Nucleotide Exchange Factor [https://en.wikipedia.org/wiki/Guanine_nucleotide_exchange_factor GEF] is recruited (like [http://www.uniprot.org/uniprot/Q07889 SOS1]), stimulating the activation of other pathways. |
| - | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue.
| + | Several others interactions have been elucidated like the capacity of the protein to dimerise proving its potential implication in the growth of [https://en.wikipedia.org/wiki/Malignancy malignant cells]. |
| | | | |
| - | <Structure load='1gri' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' />
| + | == '''Structure''' == |
| | + | Grb2 protein has a very well characterized structure. Composed of 217 amino acids organized in two chains structured in [https://en.wikipedia.org/wiki/Beta_sheet β sheets] and [https://en.wikipedia.org/wiki/Alpha_helix α helices]. |
| | + | [[Image:linearstructure.jpg|thumb|upright=5|[http://www.uniprot.org/uniprot/P62993#structure source]]] |
| | + | The protein has three main domains: |
| | + | - <scene name='75/750264/Sh3_1_58/1'>A SH3 domain (1-58) long of 58 amino acids</scene> |
| | + | - <scene name='75/750264/Sh2/1'>A SH2 domain (60-152) 93 amino acids long</scene> |
| | + | - Another <scene name='75/750264/Sh3_156_215/1'>SH3 domain (156-215)</scene> 60 amino acids long |
| | | | |
| - | == Structure == | + | <scene name='75/750264/Sh2/1'>SH2 DOMAIN</scene>: |
| - | Grb2 protein has a very well characterized structure. Composed of 217 amino acids structured in B sheets and alpha helices.
| + | |
| | | | |
| - | The protein has three main domains: | + | SH2 domain is a domain that is approximately 100 amino acids long with a very conserved structure. It has been identified in several human and rodent proteins such as [https://en.wikipedia.org/wiki/Phosphatase phosphatases], [https://en.wikipedia.org/wiki/Transcription_factor transcription factor], or [https://en.wikipedia.org/wiki/Signal_transducing_adaptor_protein adaptor] protein like Grb2. |
| - | - A SH3 domain (1-58) long of 58 amino acids | + | This domain is ubiquitous in several protein implicated in cellular signaling pathways.Typically, the SH2 domain specifically recognizes sites with '''phosphorylated tyrosines'''. SH2 can, for instance bind to the intracellular region of EGF leading in turn, to the formation of protein signalization complexes. This binding and the role of SH2 is very important in '''the conversion of an extra-cellular signal in an intra-cellular signal''' giving rise to diversified cellular responses or the expression of specific genes. It is also important to note that the SH2 domain can bind to other SH2 domains. Nevertheless, a mutation in the specific binding site of SH2 can impede the interaction of two proteins and thus the formation of a protein complex. Therefore, mutations in SH2 can give rise to cellular dysfunction and lead to several diseases. <ref>PMID: 18767163</ref> |
| - | - A SH2 domain (60-152) long od 93 amino acids | + | |
| - | - Another SH3 domain (156-215) 60 amino acids long | + | <scene name='75/750264/Sh3/1'>SH3 DOMAIN</scene>: |
| | + | |
| | + | The SH3 domain is approximately 50 amino acid long. Largely '''expressed in proteins associated with the membrane'''. The domain is made of 5 to 6 β-sheets arranged in two antiparallel β-sheets. The linking region between the two β-sheets is made of α helices. This special conformation allows the '''arrangement of a hydrophobic pocket in which the ligand can bind.''' Typically, the binding region has a '''motif rich in Prolines: PXXP'''. This binding allows the formation of multi-protein complexes involved in the translation and conversion of extra-cellular signals. The binding is thus largely involved in gene expression and protein concentration. <ref>PMID: 1279434</ref> |
| | + | |
| | + | '''ISOFORM''': |
| | + | Grb2 posses an isoform, known as '''Grb3.3'''. |
| | + | Grb3.3 is present in cells but it '''induces apoptosis'''. The isoform has a very similar structure to Grb2 but is truncated from the 60th to the 100th amino acid resulting in a degradation of the SH2 domain and a loss of functionality. <ref>PMID: 8178156</ref> |
| | + | |
| | + | == '''Function''' == |
| | + | The Grb3.3 isoform has a non-functional SH2 domain, unable to bind the phosphorylated tyrosines of its targeted protein (EGFR for instance). The inability of the molecule to transmit signal is translated by apoptosis of the cell, thus regulating growth signal. |
| | + | |
| | + | The functional isoform: Grb2, is involved in several cellular functions detailed below: |
| | + | On one hand, the SH2 domain recognizes phosphorylated residues which are mainly tyrosines. The recognized tyrosines present '''a caracteristic motif for recognition: NH2-pYXNX-COOH'''. |
| | + | |
| | + | - pY representing the phosphorylated tyrosine. |
| | + | - N for Asparagine |
| | + | - X for a random residue |
| | + | |
| | + | By the special recognition of this motif, the binding of the two molecules is very specific. These motifs are highly expressed in several cellular proteins like [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase''' Receptor Tyrosine Kinase'''] ([http://www.uniprot.org/uniprot/P00533 '''epidermal growth factor receptor'''], [http://www.uniprot.org/uniprot/P11362 '''fibroblast growth factor receptor''']) but equally in proteins that are not [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase Receptor Tyrosine Kinase] ([http://www.uniprot.org/uniprot/Q05397 f'''ocal adhesion kinase'''], [http://www.uniprot.org/uniprot/P35568 i'''nsulin receptor substrate-1''']). |
| | + | The recognition of phosphorylated tyrosine on the intracellular domain of Growth Factor Receptors by SH2 domain of Grb2 can lead to the recruitment of [http://www.uniprot.org/uniprot/Q07889 SOS-1]on the SH3 side of Grb2 able to recognize the proline rich region of [http://www.uniprot.org/uniprot/Q07889 SOS-1] protein (Son Of Sevenless). |
| | + | Following this pathway and the formation of a complex between Grb2 and [http://www.uniprot.org/uniprot/Q07889 SOS], the [http://www.uniprot.org/uniprot/P01112 RAS] protein is activated. Interestingly, [http://www.uniprot.org/uniprot/P01112 RAS] is a G-protein implicated in the activation of [http://www.uniprot.org/uniprot/P04049 RAF-1]. The latest activates the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MEK downstream cascade pathway] ([http://www.uniprot.org/uniprot/Q02750 MEK1]/ [http://www.uniprot.org/uniprot/P36507 MEK2] and [http://www.uniprot.org/uniprot/P27361 ERK1 ]/ [http://www.uniprot.org/uniprot/P28482 ERK2]) involved in the translocation of [https://en.wikipedia.org/wiki/Extracellular_signal–regulated_kinases ERK factors] from the cytosol to the nucleus for the activation of [http://www.uniprot.org/uniprot/P19419 Elk-1] and [http://www.uniprot.org/uniprot/P01106 Myc transcription Factor]. These particular [https://en.wikipedia.org/wiki/Transcription_factor transcription factors] participate in the activation of SRE containing gene leading to cellular growth. <ref> PMID: 12006650</ref> |
| | + | |
| | + | On the other hand, in [https://en.wikipedia.org/wiki/T_cell T lymphocytes], the simulation of [https://en.wikipedia.org/wiki/T-cell_receptor TCRs] induces tyrosine phosphorylation on a wide range of cellular proteins such as [http://www.uniprot.org/uniprot/P07355 p36]-[http://www.uniprot.org/uniprot/Q16539 p38] or [http://www.uniprot.org/uniprot/O43561 LAT]. |
| | + | As an example, the phosphorylated residues of [http://www.uniprot.org/uniprot/O43561 LAT] can bind the SH2 domain of Grb2 while the formation of this complex recruits on the SH3 domains of Grb2 some proteins of the [https://en.wikipedia.org/wiki/Vav_(protein) VAV family]. [http://www.uniprot.org/uniprot/P15498 '''VAV proteins] are guanine nucleotide exchange factors''' ([https://en.wikipedia.org/wiki/Guanine_nucleotide_exchange_factor GEF]) for the [https://en.wikipedia.org/wiki/GTPase GTPase proteins] of the [https://en.wikipedia.org/wiki/Rho_family_of_GTPases Rho family]. The formation of this specific complex introduces a Calcium flux and activates [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase MAP kinase] allowing [https://en.wikipedia.org/wiki/T_cell T lymphocyte] proliferation.<ref> PMID 15886116</ref> |
| | + | |
| | + | Finally, it was proven that Grb2 plays a role in '''the negative regulation of''' [http://www.uniprot.org/uniprot/P00533''' EGFR]'''. Indeed, [http://www.uniprot.org/uniprot/P22681 c-Cbl] is a protein implicated in the [http://www.uniprot.org/uniprot/O60260 '''E3] complex of''' [http://www.uniprot.org/uniprot/P00533''' EGFR] ubiquitination'''.[http://www.uniprot.org/uniprot/P22681 C-Cbl] thanks to its SH2 domain can directly bind to [http://www.uniprot.org/uniprot/P00533 EGFR] causing its degradation (Grb2 independent regulation). Or, [http://www.uniprot.org/uniprot/P22681 c-Cbl] can also indirectly bind to [http://www.uniprot.org/uniprot/P00533 EGFR] via its SH3 domain recognition by Grb2 (dependant Grb2 regulation). The direct or indirect binding of [http://www.uniprot.org/uniprot/P22681 c-Cbl] on [http://www.uniprot.org/uniprot/P00533 EGFR] induces the recruitment of enzymes that are necessary for the ubiquitination of [http://www.uniprot.org/uniprot/P00533 EGFR]. Ubiquitination being a signal for protein degradation. It is important to note that n'''egative regulation is more important when Grb2 is implicated and bound to''' [http://www.uniprot.org/uniprot/P22681 '''c-Cbl] rather than when''' [http://www.uniprot.org/uniprot/P22681 '''c-Cbl] is the only protein involved'''. <ref>PMID 10531381</ref> <ref> PMID 11823423</ref> |
| | + | |
| | + | |
| | + | |
| | + | == '''Interactions''' == |
| | + | [http://www.uniprot.org/uniprot/Q07889 '''Sos1''']: Promotes the exchange of [http://www.uniprot.org/uniprot/P01112 Ras]-bound [https://en.wikipedia.org/wiki/Guanosine_diphosphate GDP] into [https://en.wikipedia.org/wiki/Guanosine_triphosphate GTP], by promoting the [http://www.uniprot.org/uniprot/P01112 Ras] specific guanine nucleotide exchange factor activity. <ref>PMID: 10570290</ref> |
| | + | |
| | + | [http://www.uniprot.org/uniprot/P29353 '''Shc''']: [http://www.uniprot.org/uniprot/P29353 Shc] is important in the regulation of apoptosis and drug resistance in mammalian cells. It is implicated in the [https://en.wikipedia.org/wiki/Epidermal_growth_factor_receptor EGFR pathway]. <ref>PMID: 11370743</ref> |
| | + | |
| | + | [http://www.uniprot.org/uniprot/P22681 '''Cbl''']: [http://www.uniprot.org/uniprot/P22681 Cbl] is a proto oncogene protein which serves as an adaptor and a negative regulator of many signalling pathways implicated in [https://en.wikipedia.org/wiki/Cell_surface_receptor cell surface receptors] activation. <ref>PMID: 21725061</ref> |
| | + | |
| | + | [http://www.uniprot.org/uniprot/Q9UQC2 '''Gab2''']: [http://www.uniprot.org/uniprot/Q9UQC2 Gab2] acts downstream of several [https://en.wikipedia.org/wiki/Cell_surface_receptor cell surface receptors] such as [https://en.wikipedia.org/wiki/Cytokine cytokine], [https://en.wikipedia.org/wiki/Hormone hormone], cell matrix or [https://en.wikipedia.org/wiki/Growth_factor_receptor growth factor receptor]. Thus, it is implicated in many different pathways. <ref>PMID: 11782427</ref> |
| | + | |
| | + | [http://www.uniprot.org/uniprot/Q13094 '''LCP2''']: Involved in [https://en.wikipedia.org/wiki/T-cell_receptor T cell antigen receptor] mediated signaling. <ref>PMID: 10204582</ref> |
| | + | |
| | + | [http://www.uniprot.org/uniprot/P04626 '''Erbb2''']: [http://www.uniprot.org/uniprot/P04626 Erbb2] is a kinase involved in several surface receptor complexes, but need a co-receptor for ligand binding. For example, it participates in neuregulin receptor complex but it can’t bind to it on its own. <ref>PMID: 16729043</ref> |
| | + | |
| | + | [http://www.uniprot.org/uniprot/Q8WU20 '''Frs2''']: [http://www.uniprot.org/uniprot/P21802 Fibroblast growth factor receptor substrate 2] can bind to [http://www.uniprot.org/uniprot/P09769 FGR] and NGF activated receptor. They play an important role in the activation of MAPK kinase, or the phosphorylation of [http://www.uniprot.org/uniprot/P27986 PIK3R1]. <ref>PMID: 11997436</ref> |
| | | | |
| - | SH2 DOMAIN:
| + | [http://www.uniprot.org/uniprot/P35568 '''Irs1''']: [[http://www.uniprot.org/uniprot/P35568 Insulin receptor substrate 1] may mediate the control of various cellular processes by insulin. It can activate the phosphatidylinositol 3 kinase when it binds to the regulatory [http://www.uniprot.org/uniprot/P27986 p85 subunit]. <ref>PMID: 12173038</ref> |
| | | | |
| - | SH2 domain is a domain that is approximately 100 amino acids long and with a very conserved structure. Identified in in several human and rodent proteins such as phosphatases, TF, or adaptor like protein such as Grb2 for instance.
| + | [http://www.uniprot.org/uniprot/Q13480 '''Gab1''']: GRB2 associated binding protein 1, is implicated in many signalling cascades triggered by activated receptor type kinases. It is also probably involved in signalling by the epidermal growth factor receptor. <ref>PMID: 8596638</ref> |
| - | This domain is therefore ubiquitous in several cellular signaling pathways.
| + | |
| - | Typically, SH2 domain specifically recognize sites with phosphorylated tyrosine in different types of proteins.
| + | |
| - | SH2 can for instance bind to the intracellular region of EGF leading in turn, to the formation of protein signalization complexes.
| + | |
| - | This binding and the role of SH2 is thus, very important in the conversion of an extra-cellular signal in an intra-cellular signal able to give rise to diversified cellular responses or the expression of specific genes.
| + | |
| - | It is also important to note that the SH2 domain can bind to other SH2 domains. | + | |
| - | However, a mutation in the specific binding site of SH2 can impede the interaction of two proteins and thus the formation of a protein complex. Therefore, mutations in SH2 can give rise to cellular dysfunction and lead to several diseases.
| + | |
| | | | |
| - | SH3 DOMAIN:
| + | [http://www.uniprot.org/uniprot/P00533 '''EGFR''']: The epidermal growth factor receptor has a Tyrosine kinase activity and can be recognized by Grb2 thanks to its Tyrosine domains. This receptor is implicated in many pathways, such as antigen fixation on [https://en.wikipedia.org/wiki/B_cell B cells]. <ref>PMID: 11084343</ref> |
| | | | |
| - | The SH3 domain is a region of a protein that is approximately 50 amino acid long. Largely present in proteins associated to the membrane. | |
| - | The domain is made of 5 to 6 Beta-sheets arranged in two antiparallel Beta-sheets. The linking region between the two Beta-sheets can contain alpha helices. This special conformation allows the arrangement of a hydrophobic pocket in which the ligand can bind. Typically, the binding region has a motif rich in Prolines: PXXP | |
| - | This binding allows the formation of multi-proteins complexes involved in the translation of an extra-cellular signal and its conversion. The binding can thus be largely involved in gene expression and protein concentration. | |
| | | | |
| - | ISOFORM:
| + | == '''EGFR interaction''' == |
| | + | [[Image:EGFR Grb2.jpg|thumb|upright=4|[http://www.ebi.ac.uk/intact/interaction/EBI-7874813 source]]] |
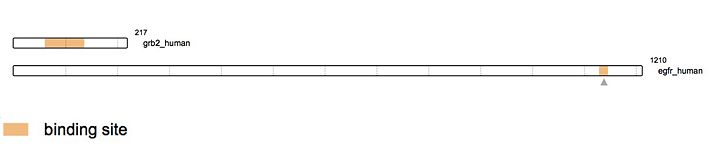
| | + | As stated earlier, Grb2 is made of an SH2 domain able to bind to [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase tyrosine kinase receptors]. Thus, Grb2 is able to bind to the activated form of the Epidermal Growth Factor Receptor ([http://www.uniprot.org/uniprot/P00533 EGFR]). [http://www.uniprot.org/uniprot/P00533 EGFR] activation mainly comes from the binding of a ligand. There is a wide range of ligands that are able to bind [http://www.uniprot.org/uniprot/P00533 EGFR] yet, the majority of the ligands come from the [https://en.wikipedia.org/wiki/ErbB ErbB family]. The most known ligands are [http://www.uniprot.org/uniprot/P01137 '''TGF-β'''] and [http://www.uniprot.org/uniprot/P01133 '''EGF''']. The binding of these latest induces [http://www.uniprot.org/uniprot/P00533 EGFR] dimerization. This '''dimerization''' activates the intracellular tyrosine kinase domain characterized by the '''autophosphorylation of tyrosines (Y992, Y1045, Y1068, Y1086 and Y1173'''). The activated form of [http://www.uniprot.org/uniprot/P00533 EGFR] then recruits Grb2. Indeed, the SH2 domain of Grb2 (from the 60th to the 152nd amino acid) binds the phosphorylated tyrosines of [http://www.uniprot.org/uniprot/P00533 EGFR] (Y1068 & Y1086). This interaction recruits [http://www.uniprot.org/uniprot/Q07889 SOS] (Son Of Sevenless) via the SH3 domain of Grb2. [http://www.uniprot.org/uniprot/Q07889 SOS] is a [https://en.wikipedia.org/wiki/Guanine_nucleotide_exchange_factor GEF protein] activating [http://www.uniprot.org/uniprot/P01112 RAS] and therefore in turn the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MAPK pathway]. <ref>PMID: 16273093</ref> |
| | + | Therefore, as this example elegantely demonstrates, Grb2 is an '''adaptor protein''' able to conduct a signal between two different proteins via its different domains. |
| | | | |
| - | Nevertheless, Grb2’s isoform is also present in the cell and induces apoptosis. This isoform has a very similar structure to Grb2 but is truncated from an SH3 domain (from the 60th amino acid to the 100th ) resulting in a degradation of its SH2 domain and therefore in a loss of functionality.
| + | <br style="clear:both" /> |
| | | | |
| - | == Function == | + | == '''Diseases''' == |
| - | The Grb3 isoform has a non-functional SH2 domain, unable to link the phosphorylated tyrosine of its targeted protein (EGFR for instance). This inability of the molecule to transmit signal is traduce by apoptosis of the cell, thus regulating the growth signal.
| + | |
| | | | |
| - | The functional isoform Grb2 is involved in several cellular functions detailed below.
| + | [https://en.wikipedia.org/wiki/Alzheimer's_disease '''Alzheimer’s Disease (AD)''']: |
| - | On one hand, the SH2 domain recognizes phosphorylated residues which are mainly tyrosines. The recognized tyrosines present a caracteristic motif for recognition : NH2-pYXNX-COOH.
| + | |
| - | - pY representing the phosphorylated tyrosine.
| + | |
| - | - N for Asparagine
| + | |
| - | - X for a random residue
| + | |
| | | | |
| - | Thus by the special recognition of this motif, the binding of the 2 molecules is very specific. These motifs are highly expressed in several cellular proteins like Receptor Tyrosine Kinase (epidermal growth factor receptor, fibroblast growth factor receptor, nerve growth factor receptor) but equally in proteins that are not RTK kinases (BCR-Ab1, focal adhesion kinase, insulin receptor substrate-1).
| + | Phenotypic changes have been identified in cortical and hippocampal neurons characteristic of AD. It seems that Grb2 is implicated in the stimulation of AD. Indeed, the proteins involved in the transduction of the signal from Grb2 to [http://www.uniprot.org/uniprot/Q07889 SOS] are altered in AD. These modifications would be at the heart of the transduction of a “derived” signal stimulating AD. <ref>PMID: 9878757</ref> |
| | | | |
| - | As an example, the SH2 domain of Grb2 recognizes an intracellular phosphorylated tyrosine. This binding, in turn, leads to the recruitment of SOS-1 via the SH3 domain of Grb2. | |
| - | Indeed, Grb2 is also made of two SH3 domains. These domains are able to recognize Proline rich region like the one of SOS-1 protein (Son Of Sevenless). | |
| - | Following this pathway and the formation of a complex between Grb2 and SOS, the RAS protein is activated. Interestingly, RAS is a g-protein implicated in the activation of Raf-1. The latest activates of the MEK downstream cascade pathway (MEK1/ MEK2 et ERK1/ ERK2) involved in the translocation of ERF factors from the cytosol to the nucleus for the activation of Elk-1 and Myc transcription Factor (TF). These particular TF participate in the activation of SRE containing gene leading to cellular growth. | |
| | | | |
| - | On the other hand, in T lymphocytes, the simulation of TCRs induces tyrosine phosphorylation on a wide range of of cellular proteins such as p36-38 or LAT.
| + | [https://en.wikipedia.org/wiki/Alzheimer's_disease '''HIV-1''']: |
| - | As an example, the phosphorylated residues of LAT can bind the SH2 domain of Grb2 while the formation of this complex recruits on the SH3 domain some proteins of the VAV family. VAV proteins are guanine nucleotide exchange factors (GEF) for the GTPase proteins of the Rho family.
| + | |
| - | This complex has for main aim to introduce a Calcium flux and the activation of MAP kinase allowing lymphocytes T proliferation.
| + | |
| | | | |
| - | Finally, it was proven that Grb2 in the negative regulation of EGFR.
| + | Grb2's isoform could have '''a simulatory effect in the retro viral infection of''' [https://en.wikipedia.org/wiki/Alzheimer's_disease '''HIV-1''']. By its essential role in the MAPK pathway, Grb3 can have effects on [https://en.wikipedia.org/wiki/Alzheimer's_disease HIV-1] infections. Indeed, the replication of the virus is activated by [https://en.wikipedia.org/wiki/T_cell Lymphocytes T] replication. Yet [https://en.wikipedia.org/wiki/T_cell Lymphocytes T]’s activation depends on the activation of the [https://en.wikipedia.org/wiki/MAPK/ERK_pathway MAPK pathway] dictated by the presence or not of Grb3 in the cell. This pathway finally activates [http://www.uniprot.org/uniprot/Q14934 NFAT], a transcription factor enhancing the [https://en.wikipedia.org/wiki/Long_terminal_repeat LTR promotor] of [https://en.wikipedia.org/wiki/Alzheimer's_disease HIV-1] leading to its replication. <ref>PMID: 10906142</ref> |
| - | Indeed, c-Cbl is a protein implicated in the E3 complex of EGFR ubiquitination, hence also its degradation. C-Cbl thanks to its SH2 domain can directly bind to EGFR causing its
| + | |
| - | == Disease ==
| + | |
| | | | |
| - | Alzheimer’s Disease (AD):
| + | == '''Relevance''' == |
| - | It seems like Grb2 is implicated in the simulation of AD. Phenotypic change have been identified in cortical and hippocampal neurons characteristic of AD.
| + | [[Image:Y160.jpg|thumb|upright=3|[http://www.nature.com/articles/ncomms8354#abstract source]]] |
| - | Indeed, the proteins implicated in the transduction of the signal from Grb2 to SOS are altered in AD. This modifications would be at the heart at the transduction of a “derived” signal stimulating AD.
| + | |
| | | | |
| - | HIV-1:
| + | Grb2 protein is especially involved in the '''setting up of cellular oncognesis''' in prostate, colon and lung cancers. This role is mainly due to its essential role in signal transduction in the [https://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase MAP kinase pathway] known to induce [https://en.wikipedia.org/wiki/Mitosis mitosis]. In this pathway, Grb2 binds to the oncogenic protein [http://www.uniprot.org/uniprot/Q07889 SOS] under its monomeric form. Yet Grb2 can also be found in its dimeric form in the cell. Dimerization of Grb2 is dependent upon several factors like the phosphorylation of <scene name='75/750264/Y160/1'>tyrosine 160</scene> or the binding of ligand on the SH2 domain of the same protein. Mainly, phosphorylation induces the dissociation of the Grb2 dimer bringing about an increase in the MAP kinase pathway by the binding of [http://www.uniprot.org/uniprot/Q07889 SOS]. The phosphorylated state of <scene name='75/750264/Y160/1'>Y160</scene> has been discovered in several pre-metastatis cancers, highly suggesting that pY160 could be a oncogenic marker in humans. A new therapeutic strategy could therefore be considered by stabilizing Grb2 in its dimeric form. This could be achieved with a protein acting as an irreversible cross-link at the interface between the two units. <ref>PMID: 26103942</ref> |
| - | Grb2 isoform could have a simulatory effect in the retro viral infection of HIV-1. | + | |
| - | By its essential role in the MAPK pathway, Grb3 can have effects on HIV-1 infections. Indeed, the replication of the virus is activated by Lymphocytes T replication. Yet lymphocytes T’s activation depend on the activation of the MAPK pathway dictated by the presence or not of grb3 in the cell. This pathway finally activates NFAT TF, a TF enhancing the LTR promotor of HIV-1 leading to its replication.
| + | |
| | | | |
| - | == Therapeutic use == | + | <br style="clear:both" /> |
| - | Grb2 protein is especially involved in the setting up of cellular oncognesis in prostate, colon and lung cancers. This role is mainly due to its essential role in signal transduction in the MAP kinase pathway known to induce mitosis.
| + | |
| - | In this pathway, GrbS binds to the oncogenic protein SOS under its monomeric form. Yet SOS can also be found in its dimeric form in the cell.
| + | |
| - | Dimerization of Grb2 is dependent upon several factors like the phosphorylation of tyrosine 160 or the binding of ligand on the SH2 domain of the same protein. Mainly, phosphorylation induce the dissociation of the Grb2 dimer induce an increase in the MAP kinase pathway activation by the binding of SOS.
| + | |
| - | The phosphorylated state of Y160 has been discovered in severa pre-metastatis cancers. This highly suggest that pY160 could be a oncogenic marker in humans.
| + | |
| - | A new therapeutic way could therefore be considered by stabilizing Grb2 in its dimeric form. This could be achieve with a protein acting as an irreversible cross-link at the interface between the 2 units.
| + | |
| | | | |
| | | | |
| - | == Relevance == | |
| | | | |
| | | | |
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | |
| | | | |
| - | </StructureSection>
| + | == '''References''' == |
| - | == References == | + | |
| | <references/> | | <references/> |
| Growth Factor Receptor Bound Protein (Grb2) is a cytosolic protein made of 217 amino acids and weighing 25,206 Da. Ubiquitously present in the cell, the protein is involved in signal transduction and plays a major role in the MAP kinase pathway. Grb2 interacts mainly with tyrosine kinases such as EGFR. When EGFR is activated by ligand binding, a Guanine Nucleotide Exchange Factor GEF is recruited (like SOS1), stimulating the activation of other pathways.
Several others interactions have been elucidated like the capacity of the protein to dimerise proving its potential implication in the growth of malignant cells.
Structure
Grb2 protein has a very well characterized structure. Composed of 217 amino acids organized in two chains structured in β sheets and α helices.
The protein has three main domains:
-
-
- Another 60 amino acids long
:
SH2 domain is a domain that is approximately 100 amino acids long with a very conserved structure. It has been identified in several human and rodent proteins such as phosphatases, transcription factor, or adaptor protein like Grb2.
This domain is ubiquitous in several protein implicated in cellular signaling pathways.Typically, the SH2 domain specifically recognizes sites with phosphorylated tyrosines. SH2 can, for instance bind to the intracellular region of EGF leading in turn, to the formation of protein signalization complexes. This binding and the role of SH2 is very important in the conversion of an extra-cellular signal in an intra-cellular signal giving rise to diversified cellular responses or the expression of specific genes. It is also important to note that the SH2 domain can bind to other SH2 domains. Nevertheless, a mutation in the specific binding site of SH2 can impede the interaction of two proteins and thus the formation of a protein complex. Therefore, mutations in SH2 can give rise to cellular dysfunction and lead to several diseases. [1]
:
The SH3 domain is approximately 50 amino acid long. Largely expressed in proteins associated with the membrane. The domain is made of 5 to 6 β-sheets arranged in two antiparallel β-sheets. The linking region between the two β-sheets is made of α helices. This special conformation allows the arrangement of a hydrophobic pocket in which the ligand can bind. Typically, the binding region has a motif rich in Prolines: PXXP. This binding allows the formation of multi-protein complexes involved in the translation and conversion of extra-cellular signals. The binding is thus largely involved in gene expression and protein concentration. [2]
ISOFORM:
Grb2 posses an isoform, known as Grb3.3.
Grb3.3 is present in cells but it induces apoptosis. The isoform has a very similar structure to Grb2 but is truncated from the 60th to the 100th amino acid resulting in a degradation of the SH2 domain and a loss of functionality. [3]
Function
The Grb3.3 isoform has a non-functional SH2 domain, unable to bind the phosphorylated tyrosines of its targeted protein (EGFR for instance). The inability of the molecule to transmit signal is translated by apoptosis of the cell, thus regulating growth signal.
The functional isoform: Grb2, is involved in several cellular functions detailed below:
On one hand, the SH2 domain recognizes phosphorylated residues which are mainly tyrosines. The recognized tyrosines present a caracteristic motif for recognition: NH2-pYXNX-COOH.
- pY representing the phosphorylated tyrosine.
- N for Asparagine
- X for a random residue
By the special recognition of this motif, the binding of the two molecules is very specific. These motifs are highly expressed in several cellular proteins like Receptor Tyrosine Kinase (epidermal growth factor receptor, fibroblast growth factor receptor) but equally in proteins that are not Receptor Tyrosine Kinase (focal adhesion kinase, insulin receptor substrate-1).
The recognition of phosphorylated tyrosine on the intracellular domain of Growth Factor Receptors by SH2 domain of Grb2 can lead to the recruitment of SOS-1on the SH3 side of Grb2 able to recognize the proline rich region of SOS-1 protein (Son Of Sevenless).
Following this pathway and the formation of a complex between Grb2 and SOS, the RAS protein is activated. Interestingly, RAS is a G-protein implicated in the activation of RAF-1. The latest activates the MEK downstream cascade pathway (MEK1/ MEK2 and ERK1 / ERK2) involved in the translocation of ERK factors from the cytosol to the nucleus for the activation of Elk-1 and Myc transcription Factor. These particular transcription factors participate in the activation of SRE containing gene leading to cellular growth. [4]
On the other hand, in T lymphocytes, the simulation of TCRs induces tyrosine phosphorylation on a wide range of cellular proteins such as p36-p38 or LAT.
As an example, the phosphorylated residues of LAT can bind the SH2 domain of Grb2 while the formation of this complex recruits on the SH3 domains of Grb2 some proteins of the VAV family. VAV proteins are guanine nucleotide exchange factors (GEF) for the GTPase proteins of the Rho family. The formation of this specific complex introduces a Calcium flux and activates MAP kinase allowing T lymphocyte proliferation.[5]
Finally, it was proven that Grb2 plays a role in the negative regulation of EGFR. Indeed, c-Cbl is a protein implicated in the E3 complex of EGFR ubiquitination.C-Cbl thanks to its SH2 domain can directly bind to EGFR causing its degradation (Grb2 independent regulation). Or, c-Cbl can also indirectly bind to EGFR via its SH3 domain recognition by Grb2 (dependant Grb2 regulation). The direct or indirect binding of c-Cbl on EGFR induces the recruitment of enzymes that are necessary for the ubiquitination of EGFR. Ubiquitination being a signal for protein degradation. It is important to note that negative regulation is more important when Grb2 is implicated and bound to c-Cbl rather than when c-Cbl is the only protein involved. [6] [7]
Interactions
Sos1: Promotes the exchange of Ras-bound GDP into GTP, by promoting the Ras specific guanine nucleotide exchange factor activity. [8]
Shc: Shc is important in the regulation of apoptosis and drug resistance in mammalian cells. It is implicated in the EGFR pathway. [9]
Cbl: Cbl is a proto oncogene protein which serves as an adaptor and a negative regulator of many signalling pathways implicated in cell surface receptors activation. [10]
Gab2: Gab2 acts downstream of several cell surface receptors such as cytokine, hormone, cell matrix or growth factor receptor. Thus, it is implicated in many different pathways. [11]
LCP2: Involved in T cell antigen receptor mediated signaling. [12]
Erbb2: Erbb2 is a kinase involved in several surface receptor complexes, but need a co-receptor for ligand binding. For example, it participates in neuregulin receptor complex but it can’t bind to it on its own. [13]
Frs2: Fibroblast growth factor receptor substrate 2 can bind to FGR and NGF activated receptor. They play an important role in the activation of MAPK kinase, or the phosphorylation of PIK3R1. [14]
Irs1: [Insulin receptor substrate 1 may mediate the control of various cellular processes by insulin. It can activate the phosphatidylinositol 3 kinase when it binds to the regulatory p85 subunit. [15]
Gab1: GRB2 associated binding protein 1, is implicated in many signalling cascades triggered by activated receptor type kinases. It is also probably involved in signalling by the epidermal growth factor receptor. [16]
EGFR: The epidermal growth factor receptor has a Tyrosine kinase activity and can be recognized by Grb2 thanks to its Tyrosine domains. This receptor is implicated in many pathways, such as antigen fixation on B cells. [17]
EGFR interaction
As stated earlier, Grb2 is made of an SH2 domain able to bind to tyrosine kinase receptors. Thus, Grb2 is able to bind to the activated form of the Epidermal Growth Factor Receptor (EGFR). EGFR activation mainly comes from the binding of a ligand. There is a wide range of ligands that are able to bind EGFR yet, the majority of the ligands come from the ErbB family. The most known ligands are TGF-β and EGF. The binding of these latest induces EGFR dimerization. This dimerization activates the intracellular tyrosine kinase domain characterized by the autophosphorylation of tyrosines (Y992, Y1045, Y1068, Y1086 and Y1173). The activated form of EGFR then recruits Grb2. Indeed, the SH2 domain of Grb2 (from the 60th to the 152nd amino acid) binds the phosphorylated tyrosines of EGFR (Y1068 & Y1086). This interaction recruits SOS (Son Of Sevenless) via the SH3 domain of Grb2. SOS is a GEF protein activating RAS and therefore in turn the MAPK pathway. [18]
Therefore, as this example elegantely demonstrates, Grb2 is an adaptor protein able to conduct a signal between two different proteins via its different domains.
Diseases
Alzheimer’s Disease (AD):
Phenotypic changes have been identified in cortical and hippocampal neurons characteristic of AD. It seems that Grb2 is implicated in the stimulation of AD. Indeed, the proteins involved in the transduction of the signal from Grb2 to SOS are altered in AD. These modifications would be at the heart of the transduction of a “derived” signal stimulating AD. [19]
HIV-1:
Grb2's isoform could have a simulatory effect in the retro viral infection of HIV-1. By its essential role in the MAPK pathway, Grb3 can have effects on HIV-1 infections. Indeed, the replication of the virus is activated by Lymphocytes T replication. Yet Lymphocytes T’s activation depends on the activation of the MAPK pathway dictated by the presence or not of Grb3 in the cell. This pathway finally activates NFAT, a transcription factor enhancing the LTR promotor of HIV-1 leading to its replication. [20]
Relevance
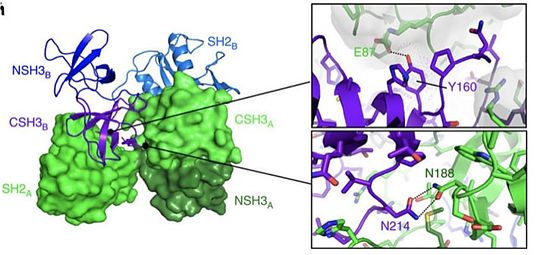
Grb2 protein is especially involved in the setting up of cellular oncognesis in prostate, colon and lung cancers. This role is mainly due to its essential role in signal transduction in the MAP kinase pathway known to induce mitosis. In this pathway, Grb2 binds to the oncogenic protein SOS under its monomeric form. Yet Grb2 can also be found in its dimeric form in the cell. Dimerization of Grb2 is dependent upon several factors like the phosphorylation of or the binding of ligand on the SH2 domain of the same protein. Mainly, phosphorylation induces the dissociation of the Grb2 dimer bringing about an increase in the MAP kinase pathway by the binding of SOS. The phosphorylated state of has been discovered in several pre-metastatis cancers, highly suggesting that pY160 could be a oncogenic marker in humans. A new therapeutic strategy could therefore be considered by stabilizing Grb2 in its dimeric form. This could be achieved with a protein acting as an irreversible cross-link at the interface between the two units. [21]
References
- ↑ Gan W, Roux B. Binding specificity of SH2 domains: insight from free energy simulations. Proteins. 2009 Mar;74(4):996-1007. doi: 10.1002/prot.22209. PMID:18767163 doi:http://dx.doi.org/10.1002/prot.22209
- ↑ Musacchio A, Noble M, Pauptit R, Wierenga R, Saraste M. Crystal structure of a Src-homology 3 (SH3) domain. Nature. 1992 Oct 29;359(6398):851-5. PMID:1279434 doi:http://dx.doi.org/10.1038/359851a0
- ↑ Fath I, Schweighoffer F, Rey I, Multon MC, Boiziau J, Duchesne M, Tocque B. Cloning of a Grb2 isoform with apoptotic properties. Science. 1994 May 13;264(5161):971-4. PMID:8178156
- ↑ Jiang X, Sorkin A. Coordinated traffic of Grb2 and Ras during epidermal growth factor receptor endocytosis visualized in living cells. Mol Biol Cell. 2002 May;13(5):1522-35. PMID:12006650 doi:http://dx.doi.org/10.1091/mbc.01-11-0552
- ↑ Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opin Immunol. 2005 Jun;17(3):267-74. PMID:15886116 doi:http://dx.doi.org/10.1016/j.coi.2005.04.003
- ↑ Yokouchi M, Kondo T, Houghton A, Bartkiewicz M, Horne WC, Zhang H, Yoshimura A, Baron R. Ligand-induced ubiquitination of the epidermal growth factor receptor involves the interaction of the c-Cbl RING finger and UbcH7. J Biol Chem. 1999 Oct 29;274(44):31707-12. PMID:10531381
- ↑ Waterman H, Katz M, Rubin C, Shtiegman K, Lavi S, Elson A, Jovin T, Yarden Y. A mutant EGF-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J. 2002 Feb 1;21(3):303-13. PMID:11823423 doi:http://dx.doi.org/10.1093/emboj/21.3.303
- ↑ Park RK, Erdreich-Epstein A, Liu M, Izadi KD, Durden DL. High affinity IgG receptor activation of Src family kinases is required for modulation of the Shc-Grb2-Sos complex and the downstream activation of the nicotinamide adenine dinucleotide phosphate (reduced) oxidase. J Immunol. 1999 Dec 1;163(11):6023-34. PMID:10570290
- ↑ Oksvold MP, Skarpen E, Wierod L, Paulsen RE, Huitfeldt HS. Re-localization of activated EGF receptor and its signal transducers to multivesicular compartments downstream of early endosomes in response to EGF. Eur J Cell Biol. 2001 Apr;80(4):285-94. PMID:11370743 doi:http://dx.doi.org/10.1078/0171-9335-00160
- ↑ Buchse T, Horras N, Lenfert E, Krystal G, Korbel S, Schumann M, Krause E, Mikkat S, Tiedge M. CIN85 interacting proteins in B cells-specific role for SHIP-1. Mol Cell Proteomics. 2011 Oct;10(10):M110.006239. doi: 10.1074/mcp.M110.006239., Epub 2011 Jul 1. PMID:21725061 doi:http://dx.doi.org/10.1074/mcp.M110.006239
- ↑ Lynch DK, Daly RJ. PKB-mediated negative feedback tightly regulates mitogenic signalling via Gab2. EMBO J. 2002 Jan 15;21(1-2):72-82. PMID:11782427
- ↑ Erdreich-Epstein A, Liu M, Kant AM, Izadi KD, Nolta JA, Durden DL. Cbl functions downstream of Src kinases in Fc gamma RI signaling in primary human macrophages. J Leukoc Biol. 1999 Apr;65(4):523-34. PMID:10204582
- ↑ Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1:2005.0008. Epub 2005 May 25. PMID:16729043 doi:http://dx.doi.org/10.1038/msb4100012
- ↑ Wong A, Lamothe B, Lee A, Schlessinger J, Lax I. FRS2 alpha attenuates FGF receptor signaling by Grb2-mediated recruitment of the ubiquitin ligase Cbl. Proc Natl Acad Sci U S A. 2002 May 14;99(10):6684-9. Epub 2002 May 7. PMID:11997436 doi:http://dx.doi.org/10.1073/pnas.052138899
- ↑ Morrison KB, Tognon CE, Garnett MJ, Deal C, Sorensen PH. ETV6-NTRK3 transformation requires insulin-like growth factor 1 receptor signaling and is associated with constitutive IRS-1 tyrosine phosphorylation. Oncogene. 2002 Aug 22;21(37):5684-95. PMID:12173038 doi:http://dx.doi.org/10.1038/sj.onc.1205669
- ↑ Holgado-Madruga M, Emlet DR, Moscatello DK, Godwin AK, Wong AJ. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996 Feb 8;379(6565):560-4. PMID:8596638 doi:http://dx.doi.org/10.1038/379560a0
- ↑ Sorkin A, McClure M, Huang F, Carter R. Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr Biol. 2000 Nov 2;10(21):1395-8. PMID:11084343
- ↑ Jones RB, Gordus A, Krall JA, MacBeath G. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature. 2006 Jan 12;439(7073):168-74. Epub 2005 Nov 6. PMID:16273093 doi:http://dx.doi.org/10.1038/nature04177
- ↑ McShea A, Zelasko DA, Gerst JL, Smith MA. Signal transduction abnormalities in Alzheimer's disease: evidence of a pathogenic stimuli. Brain Res. 1999 Jan 9;815(2):237-42. PMID:9878757
- ↑ Li X, Multon MC, Henin Y, Schweighoffer F, Venot C, Josef J, Zhou C, LaVecchio J, Stuckert P, Raab M, Mhashilkar A, Tocque B, Marasco WA. Grb3-3 is up-regulated in HIV-1-infected T-cells and can potentiate cell activation through NFATc. J Biol Chem. 2000 Oct 6;275(40):30925-33. PMID:10906142 doi:http://dx.doi.org/10.1074/jbc.M005535200
- ↑ Ahmed Z, Timsah Z, Suen KM, Cook NP, Lee GR 4th, Lin CC, Gagea M, Marti AA, Ladbury JE. Grb2 monomer-dimer equilibrium determines normal versus oncogenic function. Nat Commun. 2015 Jun 24;6:7354. doi: 10.1038/ncomms8354. PMID:26103942 doi:http://dx.doi.org/10.1038/ncomms8354
|