User:Ophelie Lefort/Sandbox
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
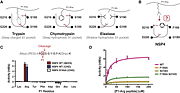
NSP4 compared to the other trypsin-like proteases has some specificity as the arginine side chain movement from the canonical "down" to the noncanonical "up" position in NSP4, which is accomplished by a rotation of the Chi2 angle by 160°, this is possible thanks to F190 which provides a hydrophobic platform that interacts with the aliphatic portion of the P1-arginine side chain. All other residue positions are preserved. The specificity for P1-arginine is conferred by H-bonds between guanidinium group and three H-bonds acceptors (S216, S192, G217). This specificity allows NSP4 to cleave citrulline, which is not cleaved by other trypsin-like proteases because of its non-charged propriety, and the impossibility to do salt-bridges. Other trypsin-like proteases are not able to cleave methylarginine neither because of a steric clash with the methyl group, which is not an issue for NSP4 since the methyl group is exposed to the solvent. The capacity to cleave these post-translationally modified arginine utility may be to act against microbial and virulence factors containing modified arginine or to interact with chemokines. <ref>Lin, S. Jack, Ken C. Dong, Charles Eigenbrot, Menno van Lookeren Campagne, and Daniel Kirchhofer. “Structures of Neutrophil Serine Protease 4 Reveal an Unusual Mechanism of Substrate Recognition by a Trypsin-Fold Protease.” Structure 22, no. 9 (September 2, 2014): 1333–40.[http://dx.doi.org/10.1016/j.str.2014.07.008]</ref> | NSP4 compared to the other trypsin-like proteases has some specificity as the arginine side chain movement from the canonical "down" to the noncanonical "up" position in NSP4, which is accomplished by a rotation of the Chi2 angle by 160°, this is possible thanks to F190 which provides a hydrophobic platform that interacts with the aliphatic portion of the P1-arginine side chain. All other residue positions are preserved. The specificity for P1-arginine is conferred by H-bonds between guanidinium group and three H-bonds acceptors (S216, S192, G217). This specificity allows NSP4 to cleave citrulline, which is not cleaved by other trypsin-like proteases because of its non-charged propriety, and the impossibility to do salt-bridges. Other trypsin-like proteases are not able to cleave methylarginine neither because of a steric clash with the methyl group, which is not an issue for NSP4 since the methyl group is exposed to the solvent. The capacity to cleave these post-translationally modified arginine utility may be to act against microbial and virulence factors containing modified arginine or to interact with chemokines. <ref>Lin, S. Jack, Ken C. Dong, Charles Eigenbrot, Menno van Lookeren Campagne, and Daniel Kirchhofer. “Structures of Neutrophil Serine Protease 4 Reveal an Unusual Mechanism of Substrate Recognition by a Trypsin-Fold Protease.” Structure 22, no. 9 (September 2, 2014): 1333–40.[http://dx.doi.org/10.1016/j.str.2014.07.008]</ref> | ||
| - | N terminus of NSP4 has been shown to be cleaved off by Cathepsin C and occurs during the translocation into the endoplasmic reticulum before the enzyme is stored in cytoplasmic granules. Natural serine protease inhibitors of NSP4 are present in human plasma and can form covalent NSP4-serpin complexes in vitro (antithrombin for example which act as a suicide substrate with NSP4). However, in vivo, antithrombin can not trap NSP4 because of the presence of other neutrophil proteases.<ref>https://doi.org/10.4049/jimmunol.1301293</ref> | + | N terminus of NSP4 has been shown to be cleaved off by Cathepsin C and occurs during the translocation into the endoplasmic reticulum before the enzyme is stored in cytoplasmic granules. Natural serine protease inhibitors of NSP4 are present in human plasma and can form covalent NSP4-serpin complexes in vitro (antithrombin for example which act as a suicide substrate with NSP4). However, in vivo, antithrombin can not trap NSP4 because of the presence of other neutrophil proteases.<ref>Perera, Natascha C., Karl-Heinz Wiesmüller, Maria Torp Larsen, Beate Schacher, Peter Eickholz, Niels Borregaard, and Dieter E. Jenne. “NSP4 Is Stored in Azurophil Granules and Released by Activated Neutrophils as Active Endoprotease with Restricted Specificity.” The Journal of Immunology 191, no. 5 (September 1, 2013) [https://doi.org/10.4049/jimmunol.1301293]</ref> |
Revision as of 08:15, 27 January 2017
Neutrophil Serine 4 or Serine Protease 57 (4Q7X)
| |||||||||||
References
[3] NSP4 is stored in azurophil granules and released by activated neutrophils as active endoprotease with restricted specificity, NCBI
[4] NSP4, an elastase-related protease in human neutrophils with arginine specificity, NCBI
[5] Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response, NCBI
[6] Role of neutrophils in innate immunity: a systems biology-level approach, NCBI
[7] Neutrophils: Their Role in Innate and Adaptive Immunity, NCBI
[8] Neutrophil extracellular traps kill bacteria, NCBI
[9] Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response, NCBI
- ↑ S.. Jack Lin, Ken C. Dong, Charles Eigenbrot, Menno van Lookeren Campagne, Daniel Kirchhofer Structures of Neutrophil Serine Protease 4 Reveal an Unusual Mechanism of Substrate Recognition by a Trypsin-Fold Protease DOI: http://dx.doi.org/10.1016/j.str.2014.07.008
- ↑ Natascha C. Perera, Karl-Heinz Wiesmüller, Maria Torp Larsen, Beate Schacher, Peter Eickholz, Niels Borregaard and Dieter E. Jenne NSP4 Is Stored in Azurophil Granules and Released by Activated Neutrophils as Active Endoprotease with Restricted Specificity DOI: https://doi.org/10.4049/jimmunol.1301293
- ↑ Lin, S. Jack, Ken C. Dong, Charles Eigenbrot, Menno van Lookeren Campagne, and Daniel Kirchhofer. “Structures of Neutrophil Serine Protease 4 Reveal an Unusual Mechanism of Substrate Recognition by a Trypsin-Fold Protease.” Structure 22, no. 9 (September 2, 2014): 1333–40.[1]
- ↑ Perera, Natascha C., Karl-Heinz Wiesmüller, Maria Torp Larsen, Beate Schacher, Peter Eickholz, Niels Borregaard, and Dieter E. Jenne. “NSP4 Is Stored in Azurophil Granules and Released by Activated Neutrophils as Active Endoprotease with Restricted Specificity.” The Journal of Immunology 191, no. 5 (September 1, 2013) [2]