User:Camille Zumstein/Sandbox
From Proteopedia
< User:Camille Zumstein(Difference between revisions)
| (13 intermediate revisions not shown.) | |||
| Line 27: | Line 27: | ||
Besides, there are six turns reported in the structure of one chain. | Besides, there are six turns reported in the structure of one chain. | ||
<br style="clear:both" /> | <br style="clear:both" /> | ||
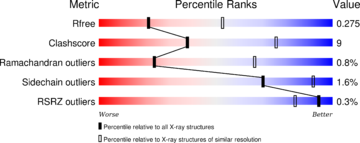
| - | The Ramachandran blot of Calcineurin [[Image:Ramachandran 4il1.jpg|thumb|left|Ramachandran Plot of 4il1]] has been obtained by [http://mordred.bioc.cam.ac.uk/~rapper/rampage2.php MolProbity of the DUke University]. It plots the torsional angles phi (φ)and psi (ψ)of the molecule and thereby represents the secondary structure. Further information about the Interpretation of this plot can be found at [[Ramachandran Plot]]. | + | The Ramachandran blot of Calcineurin [[Image:Ramachandran 4il1.jpg|thumb|left|Ramachandran Plot of 4il1]] has been obtained by [http://mordred.bioc.cam.ac.uk/~rapper/rampage2.php MolProbity of the DUke University]. It plots the torsional angles phi (φ) and psi (ψ) of the molecule and thereby represents the secondary structure. Further information about the Interpretation of this plot can be found at [[Ramachandran Plot]]. |
<br style="clear:both" /> | <br style="clear:both" /> | ||
| + | |||
== Principle of action == | == Principle of action == | ||
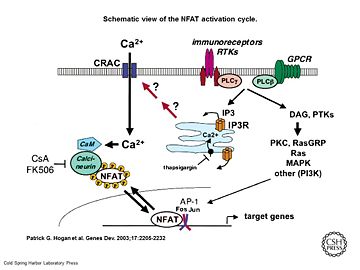
[[Image:Genes Dev. 2003 Sep 17(18) 2205-32, Figure 1.jpg|thumb|upright=2|Schematic view of the NFAT activation cycle <ref>PMID:12975316</ref>]] | [[Image:Genes Dev. 2003 Sep 17(18) 2205-32, Figure 1.jpg|thumb|upright=2|Schematic view of the NFAT activation cycle <ref>PMID:12975316</ref>]] | ||
| - | As a response of receptor tyrosine kinase [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase(RTK)] activation as well as G protein-coupled receptor [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor (GCPR)] activation the [[Phospholipase C]] [https://en.wikipedia.org/wiki/Phospholipase_C (PLC)] catalyse the hydrolysis of [https://en.wikipedia.org/wiki/Phosphatidylinositol_4,5-bisphosphate PIP2] to [https://en.wikipedia.org/wiki/Inositol_trisphosphate IP3] and [https://en.wikipedia.org/wiki/Diglyceride DAG]. IP3 activates the [[Inositol 1,4,5-Trisphosphate Receptor]] and therby leads to an increasing amount of the second Messenger | + | As a response of receptor tyrosine kinase [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase(RTK)] activation as well as G protein-coupled receptor [https://en.wikipedia.org/wiki/G_protein%E2%80%93coupled_receptor (GCPR)] activation the [[Phospholipase C]] [https://en.wikipedia.org/wiki/Phospholipase_C (PLC)] catalyse the hydrolysis of [https://en.wikipedia.org/wiki/Phosphatidylinositol_4,5-bisphosphate PIP2] to [https://en.wikipedia.org/wiki/Inositol_trisphosphate IP3] and [https://en.wikipedia.org/wiki/Diglyceride DAG]. IP3 activates the [[Inositol 1,4,5-Trisphosphate Receptor]] and therby leads to an increasing amount of the second Messenger Ca<sup>2+</sup> in the cytoplasma. |
Calcineurin is activated by [http://www.ebi.ac.uk/interpro/potm/2003_3/Page_1.htm Calmodulin], a calcium-binding protein. Calmodulin interacts with the calmodulin-binding/regulatory region of Calcineurin. That binding leads to a conformational change in the autoinhibitory domain and remove it from the active site <ref>PMID:22100452</ref>. | Calcineurin is activated by [http://www.ebi.ac.uk/interpro/potm/2003_3/Page_1.htm Calmodulin], a calcium-binding protein. Calmodulin interacts with the calmodulin-binding/regulatory region of Calcineurin. That binding leads to a conformational change in the autoinhibitory domain and remove it from the active site <ref>PMID:22100452</ref>. | ||
| Line 38: | Line 39: | ||
<br style="clear:both" /> | <br style="clear:both" /> | ||
== Binding Partners == | == Binding Partners == | ||
| - | The main partners of interaction are [https://en.wikipedia.org/wiki/Calmodulin Calmodulin],NFATc1, NFATc2 and NFATc3. | + | The main partners of interaction are [https://en.wikipedia.org/wiki/Calmodulin Calmodulin], NFATc1, NFATc2 and NFATc3. |
| - | Many of the calcineurin | + | Many of the calcineurin substrates contain a PxIxIT motif. Among them, beside the phosphorylated forms of NFAT we can also mention cAMP response element binding protein (CREB), PP1, microtubule-associated protein tau and glycogen synthase kinase-3 beta (GSK- 3)<ref>PMID: 17666045</ref><ref>PMID: 22676853</ref><ref>PMID:14701880</ref><ref>PMID: 7515479</ref>. |
| - | <scene name='75/750223/Can_fk506/1'>Calcineurin , here shown in complex with FK506 and FKPB (in pink on the model)</scene> is inhibited by the immunosuppressive drugs tacrolismus (<scene name='75/750223/Tacrolimus/1'>FK506</scene>) or cyclosporine A (CsA). CsA and conduct their therapeutic role thought binding to the [https://en.wikipedia.org/wiki/Immunophilins immunophilins] cyclophilin and FK506 binding protein (FK506BP) respectively. The complexes CsA-cyclophilin and FK506-FK506BP bind then to calcineurin in a calcium-dependent manner thus inhibiting its phosphatase activity. Therefore the addition of these drugs to lymphocytes T prevent NFAT translocation to the nucleus and the subsequent activation its target gene.That's why FK506 and CsA are use in the treatment of various immune-mediated diseases. However since calcineurin is is widely expressed in non-haemopoietic tissues like the kidney and the hearth, both drugs present a long term toxicity and can lead to deleterious effect to these Organs <ref>PMID: 8811062</ref> | + | <scene name='75/750223/Can_fk506/1'>Calcineurin, here shown in complex with FK506 and FKPB (in pink on the model)</scene> is inhibited by the immunosuppressive drugs tacrolismus (<scene name='75/750223/Tacrolimus/1'>FK506</scene>) or cyclosporine A (CsA). CsA and conduct their therapeutic role thought binding to the [https://en.wikipedia.org/wiki/Immunophilins immunophilins] cyclophilin and FK506 binding protein (FK506BP) respectively. The complexes CsA-cyclophilin and FK506-FK506BP bind then to calcineurin in a calcium-dependent manner thus inhibiting its phosphatase activity. Therefore the addition of these drugs to lymphocytes T prevent NFAT translocation to the nucleus and the subsequent activation its target gene.That's why FK506 and CsA are use in the treatment of various immune-mediated diseases. However since calcineurin is is widely expressed in non-haemopoietic tissues like the kidney and the hearth, both drugs present a long term toxicity and can lead to deleterious effect to these Organs <ref>PMID: 8811062</ref><ref>http://www.uptodate.com/contents/pharmacology-of-cyclosporine-and-tacrolimus</ref>. |
'''Cofactors''': | '''Cofactors''': | ||
| - | Calcineurin belong to the family of [https://en.wikipedia.org/wiki/Metalloprotein metalloprotein]. To conduct its activity it requires the presence of | + | Calcineurin belong to the family of [https://en.wikipedia.org/wiki/Metalloprotein metalloprotein]. To conduct its activity it requires the presence of exposing Fe<sup>3+</sup> and exposing Zn<sup>2+</sup> ions in the active site (one per subunit). Superoxide dismutase has been shown to protect calcineurin from inactivation by preventing Fe<sup>3+</sup> from oxidation. Thus after activation of calcineurin by calmodulin, the AID is displaced from the <scene name='75/750223/Catalytic_core/1'> catalytic core,with phosphate and Fe and Zn ions bound </scene> exposing Fe<sup>3+</sup> to oxidation <ref>PMID: 8837775</ref><ref>Calmodulin and Signal Transduction (p184), Linda J. Van Eldik,D. Martin Watterson (1998)</ref>. |
== Related health defects == | == Related health defects == | ||
| - | Calcineurin hyperactivation thought dysregulation of the | + | Calcineurin hyperactivation thought dysregulation of the Ca<sup>2+</sup> dynamic have been show to play a critical role in several diseases like Rheumatoid arthritis (RA), Schizophrenia ,Diabetes, Systemic Lupus Erythematosus as well as Alzheimer diseases (AD) <ref>http://www.uptodate.com/contents/pharmacology-of-cyclosporine-and-tacrolimus)</ref><ref>PMID: 12851457</ref><ref>PMID: 16988714</ref><ref>PMID:20421909</ref>. |
| - | Taking the example of AD which is a age-related memory dysfunction | + | Taking the example of AD which is a age-related memory dysfunction, it it know that in older organism the brain is less plastic due to a dysregulation of Ca<sup>2+</sup> dynamic. This in addition to the presence of oligomeric Aß is sufficient to explain an enhancement of CaN activity leading to severals symptoms like decreased neurotransmission, synaptic loss and neuroinflammation<ref>PMID:22654726</ref>. Therefore calmodulin inhibitors are potential alternatives against Alzheimer diseases. |
== Human/Rat calcineurin comparison == | == Human/Rat calcineurin comparison == | ||
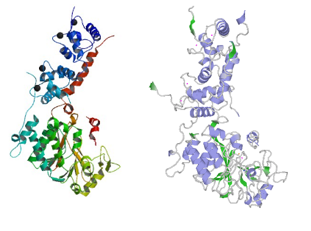
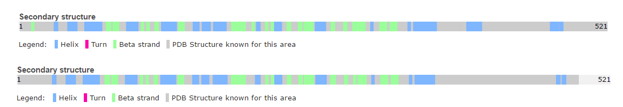
| - | [http://www.uniprot.org/uniprot/Q08209 Human] and [http://www.uniprot.org/uniprot/P63329 Rat] calcineurin have the same function and global structure [[Image:Human rat comparison.PNG|thumb|upright=4|left| Structure of rat calcineurin and human calcineurin ]]. | + | [http://www.uniprot.org/uniprot/Q08209 Human] and [http://www.uniprot.org/uniprot/P63329 Rat] calcineurin have the same function and global structure [[Image:Human rat comparison.PNG|thumb|upright=4|left| Structure of rat calcineurin and human calcineurin]]. |
| + | |||
The size (521 amino acids) and subunits of the linear structure are the same, as well as the 3D structure. | The size (521 amino acids) and subunits of the linear structure are the same, as well as the 3D structure. | ||
<br style="clear:both" /> | <br style="clear:both" /> | ||
| - | [[Image:Proteopedia.PNG|thumb|upright=4|right| Structure of human calcineurin (up) and rat calcineurin (down) ]] However, there are a few differences, such as the secondary structures. | + | [[Image:Proteopedia.PNG|thumb|upright=4|right| Structure of human calcineurin (up) and rat calcineurin (down) ]] |
| + | <br style="clear:both" /> | ||
| + | However, there are a few differences, such as the secondary structures. | ||
For instance, Human Calcineurin has one Beta strand at <scene name='75/750223/Residues_11-13_beta_strand/1'>residues 11-13</scene> whereas Rat Calcineurin has not. | For instance, Human Calcineurin has one Beta strand at <scene name='75/750223/Residues_11-13_beta_strand/1'>residues 11-13</scene> whereas Rat Calcineurin has not. | ||
| + | |||
| + | <br style="clear:both" /> | ||
Indeed, Calcineurin is a highly conserved protein from yeast to mammals. | Indeed, Calcineurin is a highly conserved protein from yeast to mammals. | ||
<br style="clear:both" /> | <br style="clear:both" /> | ||
| Line 64: | Line 70: | ||
'''Calcineurin A''' | '''Calcineurin A''' | ||
| - | This catalytic subunit is highly conserved. Nevertheless it can be up to 20% larger in lower eukaryotic species. | + | This catalytic subunit is highly conserved. Nevertheless it can be up to 20 % larger in lower eukaryotic species. |
In addition, the NH<sub>2</sub> and COOH terminals are variable among species, as well as between calcineurin A genes within the same organism. | In addition, the NH<sub>2</sub> and COOH terminals are variable among species, as well as between calcineurin A genes within the same organism. | ||
The function of these variable domains is unknown.<ref name="paper">PMID: 11015619</ref> | The function of these variable domains is unknown.<ref name="paper">PMID: 11015619</ref> | ||
Current revision
Rat Calcineurin
| |||||||||||