Pyruvate phosphate dikinase
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
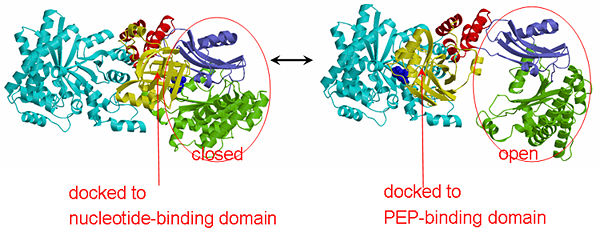
'''The His-domain in the two conformational states of PPDK. His455 is shown in blue spheres:'''[[Image:two_cond.jpg|left|600px]]<br><br><br> | '''The His-domain in the two conformational states of PPDK. His455 is shown in blue spheres:'''[[Image:two_cond.jpg|left|600px]]<br><br><br> | ||
| - | + | <div style="float:left;padding:3px;"> | |
| - | <html5media width="360" frameborder="0" allowfullscreen>https://www.youtube.com/embed/hxuBouGfs_4</html5media> | + | <html5media width="360" frameborder="0" allowfullscreen>https://www.youtube.com/embed/hxuBouGfs_4</html5media><br> |
| - | + | You may also [http://youtu.be/hxuBouGfs_4 download] the full High Resolution video. | |
| - | + | </div> | |
| - | + | ||
| - | + | ||
| - | + | ||
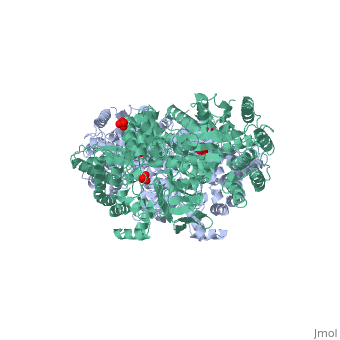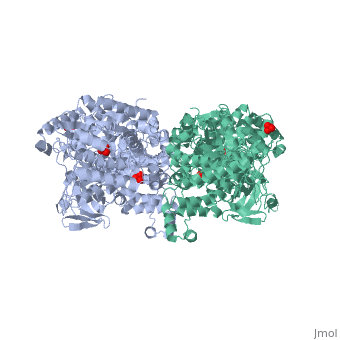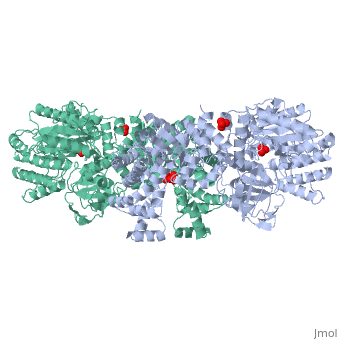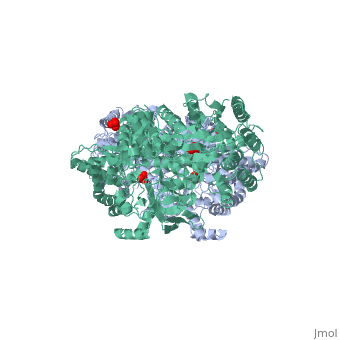
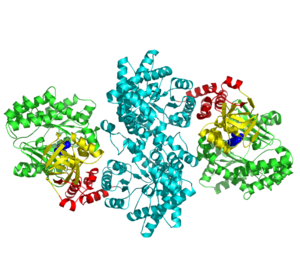
'''''The movie''' depicts the catalytic reaction involving three in-line phosphotransfers and the accompanied protein conformational transitions. This is a model based on crystal structures of PPDK from '''Clostridium symbiosum''' in the two extreme conformational states shown to the left and of complexes bound to substrate analogs, phosphonopyruvate and 5'-adenylyl-β,γ-imidodiphosphate (AMPPNP). The nucleotide binding subdomains are colored green and blue. The PEP binding domain is colored cyan. The His-domain is colored yellow, and the linker segments that connect the His-domain to the partner domains are colored red. Ligands and the catalytic histidine are depicted in stick models with the atomic color scheme: Carbon – gray, Nitrogen – blue, Oxygen – red, Phosphorous – green, Magnesium – magenta. Note that the reaction pregresses in the movie in the reverse direction; steps 3 and 2 occur first followed by step 1. The movie was created by Kap Lim and osnat Herzberg''<br> | '''''The movie''' depicts the catalytic reaction involving three in-line phosphotransfers and the accompanied protein conformational transitions. This is a model based on crystal structures of PPDK from '''Clostridium symbiosum''' in the two extreme conformational states shown to the left and of complexes bound to substrate analogs, phosphonopyruvate and 5'-adenylyl-β,γ-imidodiphosphate (AMPPNP). The nucleotide binding subdomains are colored green and blue. The PEP binding domain is colored cyan. The His-domain is colored yellow, and the linker segments that connect the His-domain to the partner domains are colored red. Ligands and the catalytic histidine are depicted in stick models with the atomic color scheme: Carbon – gray, Nitrogen – blue, Oxygen – red, Phosphorous – green, Magnesium – magenta. Note that the reaction pregresses in the movie in the reverse direction; steps 3 and 2 occur first followed by step 1. The movie was created by Kap Lim and osnat Herzberg''<br> | ||
<br> | <br> | ||
Revision as of 15:08, 6 August 2017
Pyruvate Phosphate Dikinase - a Molecular Machine
| |||||||||||
3D Structures of PPDK
Updated on 06-August-2017
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Osnat Herzberg, Michal Harel, Jaime Prilusky, Alexander Berchansky, Dan Bolser, Karl Oberholser, David Canner, Eran Hodis