We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Reverse transcriptase
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
==Function== | ==Function== | ||
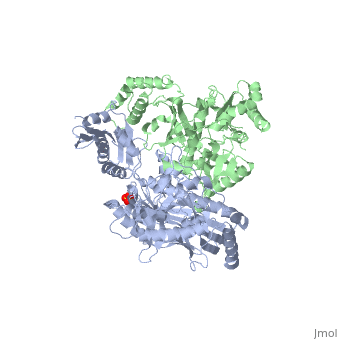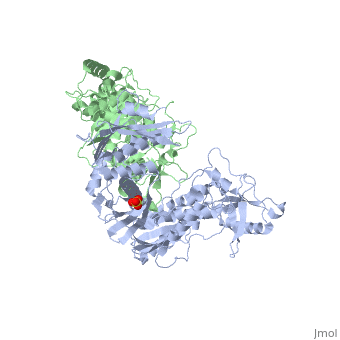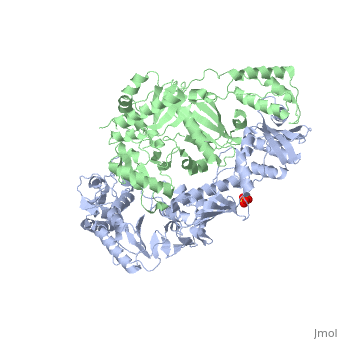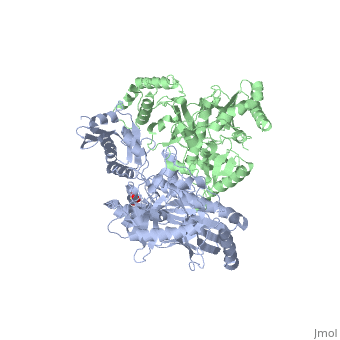
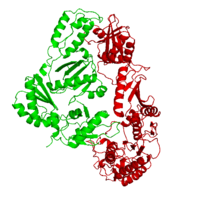
| - | As a RNA-dependent DNA Polymerase, Reverse Transcriptase is able to recognize the initial RNA, transcribe it to ssDNA, cleave the remaining RNA and then build up the dsDNA. To do this the protein has two active catalytic zones. Chain A has the <scene name='Reverse_transcriptase/Fingers/4'>Polymerase active site</scene> that consist of two ''finger-like'' domains: one of them recognizes the initial nucleic acid by h-bond interactions with phosphate groups of the side chains, then both domains make a conformational change closing the recognition hole to allow the second domain with the support a <scene name='Reverse_transcriptase/Magnesium/2'>Magnesium ion</scene> coordination system to begin the transcription process adding the specific DNA nucleotides. This change is allowed by a <scene name='Reverse_transcriptase/Flexible/2'>flexible zone</scene> between the two previous domains; it is used as a common pharmaceutical target site in order to prevent the change and therefore inhibit activity. This zone is the only zone of Chain A that has non-conserved aminoacids, giving the virus more drug resistance<ref>Consurf Data Base DOI: 10.1002/ijch.201200096</ref> [http://consurfdb.tau.ac.il/chain_selection.php?pdb_ID=1JLB Link to Consurf Data Base for PDB Entry: 1JLB]. | + | As a RNA-dependent DNA Polymerase, Reverse Transcriptase is able to recognize the initial RNA, transcribe it to ssDNA, cleave the remaining RNA and then build up the dsDNA. To do this the protein has two active catalytic zones. Chain A has the <scene name='Reverse_transcriptase/Fingers/4'>Polymerase active site</scene> that consist of two ''finger-like'' domains: one of them recognizes the initial nucleic acid by h-bond interactions with phosphate groups of the side chains, then both domains make a conformational change closing the recognition hole to allow the second domain with the support a <scene name='Reverse_transcriptase/Magnesium/2'>Magnesium ion</scene> coordination system to begin the transcription process adding the specific DNA nucleotides. This change is allowed by a <scene name='Reverse_transcriptase/Flexible/2'>flexible zone</scene> between the two previous domains; it is used as a common pharmaceutical target site in order to prevent the change and therefore inhibit activity. This zone is the only zone of Chain A that has non-conserved aminoacids, giving the virus more drug resistance<ref>Consurf Data Base DOI: 10.1002/ijch.201200096</ref> |
| + | |||
| + | <ref>[http://dx.doi.org/10.1002/ijch.201200096 DOI: 10.1002/ijch.201200096]</ref | ||
| + | |||
| + | [http://consurfdb.tau.ac.il/chain_selection.php?pdb_ID=1JLB Link to Consurf Data Base for PDB Entry: 1JLB]. | ||
As the same rate that the polymerization process occurs, the other active site known as the <scene name='Reverse_transcriptase/Rnase/2'>Ribonuclease H domain</scene> cleaves RNA, releasing the ssDNA that comes again through the Polymerase active site to become dsDNA (all this with a <scene name='Reverse_transcriptase/Magnesium2/2'>second Magnesium</scene> coordinative system, that allows non-specific recognition, just with phosphates). Finally, Chain B, despite the similar aminoacid sequence with Chain A, has no enzymatic activity; its function is possibly to stabilize and interact with both active sites by varying the length between them in order to synchronize both functions. | As the same rate that the polymerization process occurs, the other active site known as the <scene name='Reverse_transcriptase/Rnase/2'>Ribonuclease H domain</scene> cleaves RNA, releasing the ssDNA that comes again through the Polymerase active site to become dsDNA (all this with a <scene name='Reverse_transcriptase/Magnesium2/2'>second Magnesium</scene> coordinative system, that allows non-specific recognition, just with phosphates). Finally, Chain B, despite the similar aminoacid sequence with Chain A, has no enzymatic activity; its function is possibly to stabilize and interact with both active sites by varying the length between them in order to synchronize both functions. | ||
Revision as of 13:45, 27 September 2017
| |||||||||||
3D Structures of Reverse transcriptase
Updated on 27-September-2017
See Also
- Reverse Transcriptase at Wikipedia
- Molecule of the Month (09/2002) at RCSB Protein Data Bank
- List of Reverse Transcriptase articles at Proteopedia and at RCSB Protein Data Bank
- Model of Reverse Transcriptase as one of the CBI Molecules on the Molecular Playground
- See Transcription for additional Proteopedia articles on the subject.
- For additional information, see: Human Immunodeficiency Virus
- For additional information, see: Transcription and RNA Processing
References
- ↑ Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992 Jun 26;256(5065):1783-90. PMID:1377403 doi:[http://dx.doi.org/10.1126/science.1377403 http://dx.doi.org/10.1126/science.1377403
- ↑ Consurf Data Base DOI: 10.1002/ijch.201200096
- ↑ DOI: 10.1002/ijch.201200096</ref Link to Consurf Data Base for PDB Entry: 1JLB. As the same rate that the polymerization process occurs, the other active site known as the cleaves RNA, releasing the ssDNA that comes again through the Polymerase active site to become dsDNA (all this with a coordinative system, that allows non-specific recognition, just with phosphates). Finally, Chain B, despite the similar aminoacid sequence with Chain A, has no enzymatic activity; its function is possibly to stabilize and interact with both active sites by varying the length between them in order to synchronize both functions. This is the most general idea of the mechanism of action of Reverse Transcriptase; however the process remains unclear and new approaches are being reported <ref> PMID: 18464735</li></ol></ref>
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Daniel Moyano-Marino, Joel L. Sussman, Alexander Berchansky, David Canner, Amol Kapoor, Jaime Prilusky, Brian Foley, Lynmarie K Thompson, Eric Martz