We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Reverse transcriptase
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
==Function== | ==Function== | ||
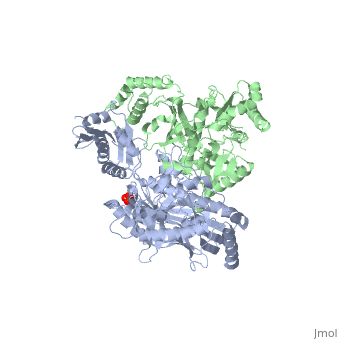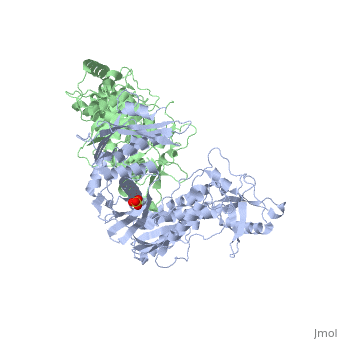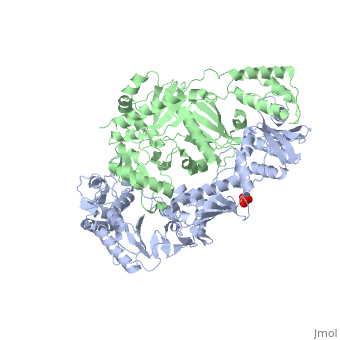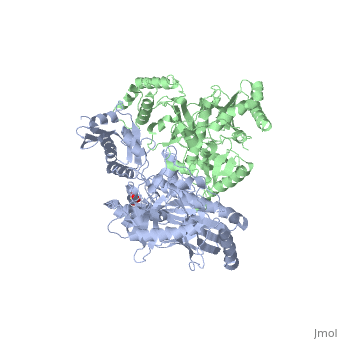
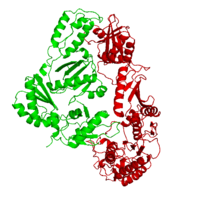
| - | As a RNA-dependent DNA Polymerase, Reverse Transcriptase is able to recognize the initial RNA, transcribe it to ssDNA, cleave the remaining RNA and then build up the dsDNA. To do this the protein has two active catalytic zones. Chain A has the <scene name='Reverse_transcriptase/Fingers/4'>Polymerase active site</scene> that consist of two ''finger-like'' domains: one of them recognizes the initial nucleic acid by h-bond interactions with phosphate groups of the side chains, then both domains make a conformational change closing the recognition hole to allow the second domain with the support a <scene name='Reverse_transcriptase/Magnesium/2'>Magnesium ion</scene> coordination system to begin the transcription process adding the specific DNA nucleotides. This change is allowed by a <scene name='Reverse_transcriptase/Flexible/2'>flexible zone</scene> between the two previous domains; it is used as a common pharmaceutical target site in order to prevent the change and therefore inhibit activity. This zone is the only zone of Chain A that has non-conserved aminoacids, giving the virus more drug resistance | + | As a RNA-dependent DNA Polymerase, Reverse Transcriptase is able to recognize the initial RNA, transcribe it to ssDNA, cleave the remaining RNA and then build up the dsDNA. To do this the protein has two active catalytic zones. Chain A has the <scene name='Reverse_transcriptase/Fingers/4'>Polymerase active site</scene> that consist of two ''finger-like'' domains: one of them recognizes the initial nucleic acid by h-bond interactions with phosphate groups of the side chains, then both domains make a conformational change closing the recognition hole to allow the second domain with the support a <scene name='Reverse_transcriptase/Magnesium/2'>Magnesium ion</scene> coordination system to begin the transcription process adding the specific DNA nucleotides. This change is allowed by a <scene name='Reverse_transcriptase/Flexible/2'>flexible zone</scene> between the two previous domains; it is used as a common pharmaceutical target site in order to prevent the change and therefore inhibit activity. This zone is the only zone of Chain A that has non-conserved aminoacids, giving the virus more drug resistance |
| - | <ref>[http://dx.doi.org/10.1002/ijch.201200096 DOI: 10.1002/ijch.201200096]</ref> | + | <ref>[http://dx.doi.org/10.1002/ijch.201200096 Consurf Data Base DOI: 10.1002/ijch.201200096]</ref> |
[http://consurfdb.tau.ac.il/chain_selection.php?pdb_ID=1JLB Link to Consurf Data Base for PDB Entry: 1JLB]. | [http://consurfdb.tau.ac.il/chain_selection.php?pdb_ID=1JLB Link to Consurf Data Base for PDB Entry: 1JLB]. | ||
Revision as of 13:48, 27 September 2017
| |||||||||||
3D Structures of Reverse transcriptase
Updated on 27-September-2017
See Also
- Reverse Transcriptase at Wikipedia
- Molecule of the Month (09/2002) at RCSB Protein Data Bank
- List of Reverse Transcriptase articles at Proteopedia and at RCSB Protein Data Bank
- Model of Reverse Transcriptase as one of the CBI Molecules on the Molecular Playground
- See Transcription for additional Proteopedia articles on the subject.
- For additional information, see: Human Immunodeficiency Virus
- For additional information, see: Transcription and RNA Processing
References
- ↑ Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992 Jun 26;256(5065):1783-90. PMID:1377403 doi:[http://dx.doi.org/10.1126/science.1377403 http://dx.doi.org/10.1126/science.1377403
- ↑ Consurf Data Base DOI: 10.1002/ijch.201200096
- ↑ Abbondanzieri EA, Bokinsky G, Rausch JW, Zhang JX, Le Grice SF, Zhuang X. Dynamic binding orientations direct activity of HIV reverse transcriptase. Nature. 2008 May 8;453(7192):184-9. PMID:18464735 doi:10.1038/nature06941
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Daniel Moyano-Marino, Joel L. Sussman, Alexander Berchansky, David Canner, Amol Kapoor, Jaime Prilusky, Brian Foley, Lynmarie K Thompson, Eric Martz