We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Pyruvate Kinase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | ||
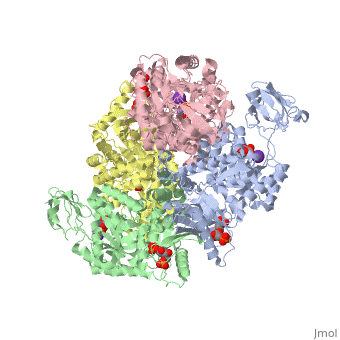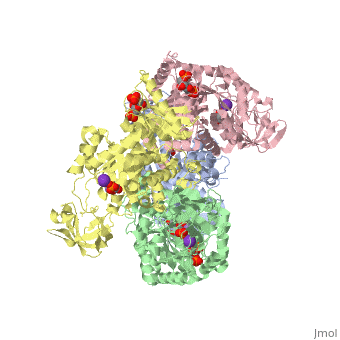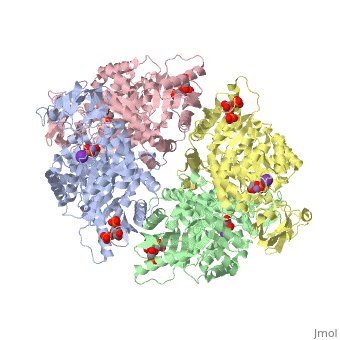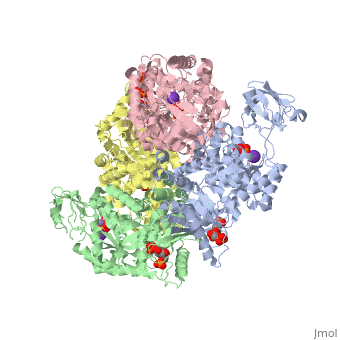
| + | <StructureSection load='2vgb' size='350' side='right' scene='' caption='Human pyruvate kinase tetramer complex with fructose diphosphate, phosphoglycolic acid, Mn+2 and K+ (purple) ions (PDB code [[2vgb]])'> | ||
[[Pyruvate Kinase]] is an enzyme that is involved in glycolysis. Pyruvate kinase’s function is to catalyze the last step of glycolysis; thereby, generating the second ATP of glycolysis and pyruvate. It is able to catalyze this step by transferring the phosphate group from phosphoenolpyruvate (PEP) to ADP <ref>{{book |author=Voet, Donald; Voet, Judith C.; Pratt, Charlotte W.|title=Fundamentals of Biochemistry: Life at the Molecular Level|edition= 3|pages=501|}}</ref>. See [[Glycolysis Enzymes]]. | [[Pyruvate Kinase]] is an enzyme that is involved in glycolysis. Pyruvate kinase’s function is to catalyze the last step of glycolysis; thereby, generating the second ATP of glycolysis and pyruvate. It is able to catalyze this step by transferring the phosphate group from phosphoenolpyruvate (PEP) to ADP <ref>{{book |author=Voet, Donald; Voet, Judith C.; Pratt, Charlotte W.|title=Fundamentals of Biochemistry: Life at the Molecular Level|edition= 3|pages=501|}}</ref>. See [[Glycolysis Enzymes]]. | ||
| Line 41: | Line 42: | ||
Red blood cells, in a state of pyruvate kinase deficiency, rapidly become deficient in ATP and can undergo hemolysis.This is transmitted as an autosomal recessive trit. The severity of hemolysis is extremely variable such as a mild case to life-threatening neonatal anaemia requiring transfusions. Over one hundred eighty different mutations have been discovered in relation to this deficiency with most being autosomal recessive, but a few strands are autosomal dominant. The deficiency causes red blood cells to deform into echinocytes on peripheral blood smears. This causes the buildup of reaction intermediates which can also increase the level of 2,3-bisphosphoglycerate in the cells. This causes a rightward shift in the hemoglobin oxygen saturation curve, which means that there is a decreased oxygen affinity for the hemoglobin and earlier oxygen unloading than under normal conditions | Red blood cells, in a state of pyruvate kinase deficiency, rapidly become deficient in ATP and can undergo hemolysis.This is transmitted as an autosomal recessive trit. The severity of hemolysis is extremely variable such as a mild case to life-threatening neonatal anaemia requiring transfusions. Over one hundred eighty different mutations have been discovered in relation to this deficiency with most being autosomal recessive, but a few strands are autosomal dominant. The deficiency causes red blood cells to deform into echinocytes on peripheral blood smears. This causes the buildup of reaction intermediates which can also increase the level of 2,3-bisphosphoglycerate in the cells. This causes a rightward shift in the hemoglobin oxygen saturation curve, which means that there is a decreased oxygen affinity for the hemoglobin and earlier oxygen unloading than under normal conditions | ||
<ref>PMID:17360088</ref>. | <ref>PMID:17360088</ref>. | ||
| - | + | </StructureSection> | |
==3D structures of pyruvate kinase== | ==3D structures of pyruvate kinase== | ||
Revision as of 17:45, 29 September 2017
| |||||||||||
3D structures of pyruvate kinase
Updated on 29-September-2017
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc., 2008, 501-503.
- ↑ authors, The scop. "Structural Classification of Proteins". 2009. 2/26 2010. <http://scop.berkeley.edu/data/scop.b.c.jh.b.b.d.html>.
- ↑ authors, The scop. "Structural Classification of Proteins". 2009. 2/26 2010. <http://scop.berkeley.edu/data/scop.b.c.jh.b.b.d.html>.
- ↑ Robergs, Robert. "Exercise-Induced Metabolic Acidosis: Where do the Protons come from?". 2009. 2/27 2010. <http://www.sportsci.org/jour/0102/rar.htm>.
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc., 2008, 501-503.
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc., 2008, 501-503.
- ↑ Dann LG, Britton HG. Kinetics and mechanism of action of muscle pyruvate kinase. Biochem J. 1978 Jan 1;169(1):39-54. PMID:629752
- ↑ Mattevi A, Bolognesi M, Valentini G. The allosteric regulation of pyruvate kinase. FEBS Lett. 1996 Jun 24;389(1):15-9. PMID:8682196
- ↑ Jurica MS, Mesecar A, Heath PJ, Shi W, Nowak T, Stoddard BL. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure. 1998 Feb 15;6(2):195-210. PMID:9519410
- ↑ Oria-Hernandez J, Cabrera N, Perez-Montfort R, Ramirez-Silva L. Pyruvate kinase revisited: the activating effect of K+. J Biol Chem. 2005 Nov 11;280(45):37924-9. Epub 2005 Sep 7. PMID:16147999 doi:10.1074/jbc.M508490200
- ↑ Dann LG, Britton HG. Kinetics and mechanism of action of muscle pyruvate kinase. Biochem J. 1978 Jan 1;169(1):39-54. PMID:629752
- ↑ Oria-Hernandez J, Cabrera N, Perez-Montfort R, Ramirez-Silva L. Pyruvate kinase revisited: the activating effect of K+. J Biol Chem. 2005 Nov 11;280(45):37924-9. Epub 2005 Sep 7. PMID:16147999 doi:10.1074/jbc.M508490200
- ↑ Zanella A, Fermo E, Bianchi P, Chiarelli LR, Valentini G. Pyruvate kinase deficiency: the genotype-phenotype association. Blood Rev. 2007 Jul;21(4):217-31. Epub 2007 Mar 13. PMID:17360088 doi:10.1016/j.blre.2007.01.001
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Keegan Gelvoria, David Canner, Ann Taylor, Andrew Alexander