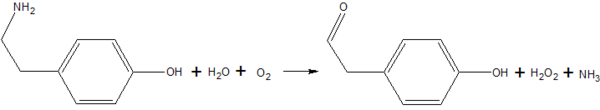
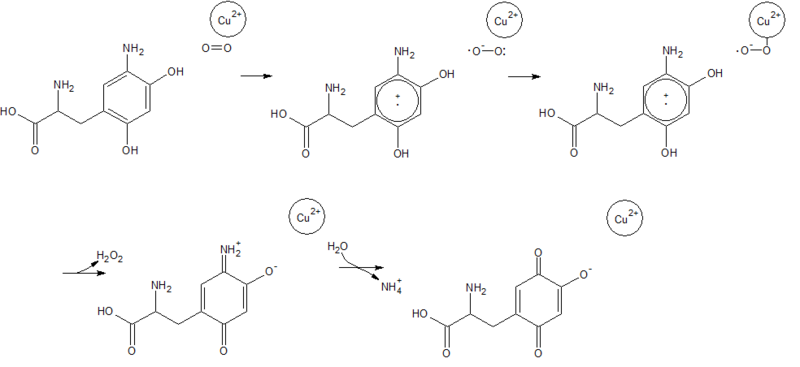
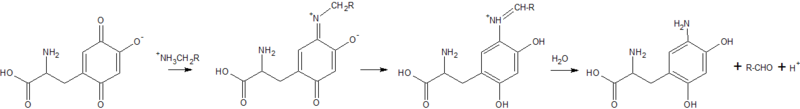Copper Amine Oxidase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | __TOC__ | ||
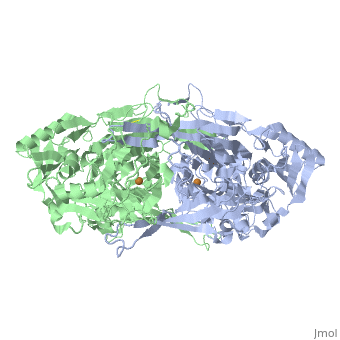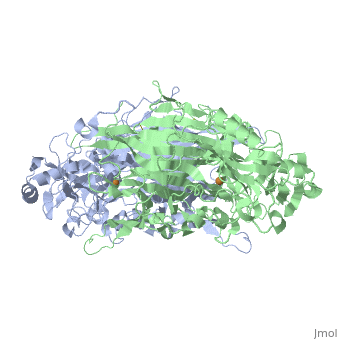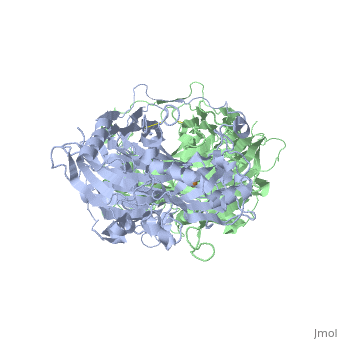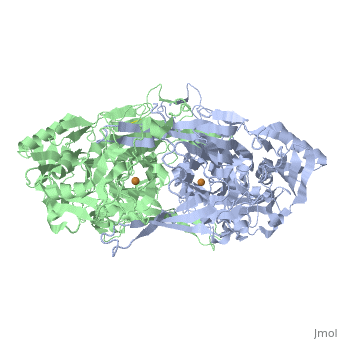
<StructureSection load='2d1w' size='350' side='right' caption='Copper amine oxidase dimer showing Cu+2 (orange) complex with tyramine (PDB code [[2d1w]]).' scene=''> | <StructureSection load='2d1w' size='350' side='right' caption='Copper amine oxidase dimer showing Cu+2 (orange) complex with tyramine (PDB code [[2d1w]]).' scene=''> | ||
Revision as of 14:52, 19 October 2017
| |||||||||||
3D structures of copper amine oxidase
Updated on 19-October-2017
Additional Resources
References
- ↑ 1.0 1.1 Murakawa T, Okajima T, Kuroda S, Nakamoto T, Taki M, Yamamoto Y, Hayashi H, Tanizawa K. Quantum mechanical hydrogen tunneling in bacterial copper amine oxidase reaction. Biochem Biophys Res Commun. 2006 Apr 7;342(2):414-23. Epub 2006 Feb 8. PMID:16487484 doi:10.1016/j.bbrc.2006.01.150
- ↑ Parsons MR, Convery MA, Wilmot CM, Yadav KD, Blakeley V, Corner AS, Phillips SE, McPherson MJ, Knowles PF. Crystal structure of a quinoenzyme: copper amine oxidase of Escherichia coli at 2 A resolution. Structure. 1995 Nov 15;3(11):1171-84. PMID:8591028
- ↑ Mure M, Mills SA, Klinman JP. Catalytic mechanism of the topa quinone containing copper amine oxidases. Biochemistry. 2002 Jul 30;41(30):9269-78. PMID:12135347
- ↑ 4.0 4.1 Grant KL, Klinman JP. Evidence that both protium and deuterium undergo significant tunneling in the reaction catalyzed by bovine serum amine oxidase. Biochemistry. 1989 Aug 8;28(16):6597-605. PMID:2790014
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Raymond Lyle, Alexander Berchansky, OCA, Jaime Prilusky