Adenylosuccinate Synthetase
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
==Structure== | ==Structure== | ||
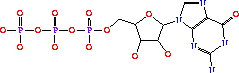
| - | The AdSS enzyme is a dimer consisting of two identical monomeric subunits. The main structural component of each monomer is a centrally located beta sheet that is comprised of 10 strands. Nine of the 10 strands are parallel while the 10th strand is anti-parallel with respect to the other 9 strands. There are also several other secondary structures including 2 small 3/10 helices and two anti-parallel sheets consisting of 2 and 3 strands respectively. Additionally there are 11 alpha helices.<ref name=crystal>PMID:8244965</ref> AdSS has three major binding sites, one for GTP, one for IMP and its active site. The active site, | + | The AdSS enzyme is a dimer consisting of two identical monomeric subunits. The main structural component of each monomer is a centrally located beta sheet that is comprised of 10 strands. Nine of the 10 strands are parallel while the 10th strand is anti-parallel with respect to the other 9 strands. There are also several other secondary structures including 2 small 3/10 helices and two anti-parallel sheets consisting of 2 and 3 strands respectively. Additionally there are 11 alpha helices.<ref name=crystal>PMID:8244965</ref> AdSS has three major binding sites, one for GTP, one for IMP and its active site. The active site, ligand molecules in orange can be seen <scene name='38/382959/Cv/3'>here</scene>. |
The major fold of this protein is unique and has not been documented in any other type of protein. The active site itself consists of a crevice consisting of Gly12, Gly15, Gly17, Lys18, Ile19, and Lys331. The other side of the crevice contains Lysine 140 and Arg147, which are located in the region between the two monomers. Asp231 is bonded to Lys140 in a salt bridge and its carbonyl atom is hydrogen bonded to Arg147. Also, none of cysteine residues are bound to each other in disulphide bonds.<ref name=crystal /> | The major fold of this protein is unique and has not been documented in any other type of protein. The active site itself consists of a crevice consisting of Gly12, Gly15, Gly17, Lys18, Ile19, and Lys331. The other side of the crevice contains Lysine 140 and Arg147, which are located in the region between the two monomers. Asp231 is bonded to Lys140 in a salt bridge and its carbonyl atom is hydrogen bonded to Arg147. Also, none of cysteine residues are bound to each other in disulphide bonds.<ref name=crystal /> | ||
Revision as of 13:26, 25 April 2018
| |||||||||||
3D structures of Adenylosuccinate Synthetase
Updated on 25-April-2018
References
- ↑ http://www.pdb.org/pdb/explore/explore.do?structureId=3HID
- ↑ Mukhopadhyay RP, Chandra AL. Keratinase of a streptomycete. Indian J Exp Biol. 1990 Jun;28(6):575-7. PMID:1698173
- ↑ Pierloot RA. The treatment of psychosomatic disorders by the general practitioner. Int J Psychiatry Med. 1977-1978;8(1):43-51. PMID:649264
- ↑ 4.0 4.1 Poland BW, Silva MM, Serra MA, Cho Y, Kim KH, Harris EM, Honzatko RB. Crystal structure of adenylosuccinate synthetase from Escherichia coli. Evidence for convergent evolution of GTP-binding domains. J Biol Chem. 1993 Dec 5;268(34):25334-42. PMID:8244965
- ↑ Ware JD, Bellini WJ, Ash RJ. Metabolic characteristics of cells infected with a herpesvirus of turkeys. J Natl Cancer Inst. 1975 Dec;55(6):1379-82. PMID:1548
- ↑ Katsarkas A, Kirkham TH. Paroxysmal positional vertigo--a study of 255 cases. J Otolaryngol. 1978 Aug;7(4):320-30. PMID:691098
- ↑ Van der Weyden MB, Kelly WN. Human adenylosuccinate synthetase. Partial purification, kinetic and regulatory properties of the enzyme from placenta. J Biol Chem. 1974 Nov 25;249(22):7282-9. PMID:4436310
- ↑ Bates PC, Millward DJ. Muscle growth and protein turnover in a fast growing rat strain. Proc Nutr Soc. 1978 May;37(1):19A. PMID:662843
- ↑ Iancu CV, Borza T, Choe JY, Fromm HJ, Honzatko RB. Recombinant mouse muscle adenylosuccinate synthetase: overexpression, kinetics, and crystal structure. J Biol Chem. 2001 Nov 9;276(45):42146-52. Epub 2001 Sep 17. PMID:11560929 doi:10.1074/jbc.M106294200
- ↑ Tyagi AK, Cooney DA. Identification of the antimetabolite of L-alanosine, L-alanosyl-5-amino-4-imidazolecarboxylic acid ribonucleotide, in tumors and assessment of its inhibition of adenylosuccinate synthetase. Cancer Res. 1980 Dec;40(12):4390-7. PMID:7438071
- ↑ Weber G. Enzymes of purine metabolism in cancer. Clin Biochem. 1983 Feb;16(1):57-63. PMID:6861338
Proteopedia Page Contributors and Editors (what is this?)
Aaron Smith, Michal Harel, Alexander Berchansky, David Canner, Andrea Gorrell