User:Alisa Cario
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
== '''Introduction and Background''' == | == '''Introduction and Background''' == | ||
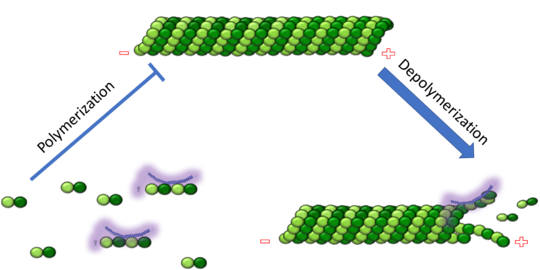
| - | The cytoskeleton brings structure to the cell, is integral in cell division, and aids in migration. One of the constitutive parts of the cytoskeleton are microtubules. Microtubules are polymerized from tubulin subunits that incorporate into a hollow cylindrical structure linked together by lateral and vertical hydrogen bonds. Each tubulin subunit is made from a heterodimer of alpha and beta tubulin. Both alpha and beta tubulin are known to bind to | + | The cytoskeleton brings structure to the cell, is integral in cell division, and aids in migration. One of the constitutive parts of the cytoskeleton are microtubules. Microtubules are polymerized from tubulin subunits that incorporate into a hollow cylindrical structure linked together by lateral and vertical hydrogen bonds. Each tubulin subunit is made from a heterodimer of alpha and beta tubulin. Both alpha and beta tubulin are known to bind to GTP. However, only the beta subunit hydrolyzes GTP, which occurs once incorporated into microtubules. The beta tubulin hydrolysis of GTP to GDP is known to destabilize microtubules. Microtubules have polarity where one end of microtubule, called the plus end, has a greater affinity to add tubulin subunits than the other end, called the minus end. Microtubules are inherently dynamic, going through periods of depolymerization, known as catastrophe, and then return to polymerization, known as rescue. Microtubules can also go through a process called tread milling, where the length of the microtubule does not change but the rate of polymerization at the plus end equals the rate of depolymerization at the minus end. There are a number of microtubule associated proteins (MAPs) that are known to regulate the dynamics of microtubules. Some examples of this include MAPTau, MAP2 and stathmin. See [[beta tubulin]], [[tau]]. |
[[Image:Stathmin_figure_cario1.png|center|thumb| upright=3| Figure XXX. Stathmin in purple can bind to tubulin dimers to prevent polymerization or to microtubules to increase catastrophe ]] | [[Image:Stathmin_figure_cario1.png|center|thumb| upright=3| Figure XXX. Stathmin in purple can bind to tubulin dimers to prevent polymerization or to microtubules to increase catastrophe ]] | ||
| Line 57: | Line 57: | ||
The C-terminal region, also known as the interaction domain, is known to sequester tubulin heterodimers. This region is comprised of a coiled-coil alpha helix. This region of stathmin is known to bind to helix 10 of alpha tubulin. Helix 10 of alpha tubulin is thought to be important for incorporation into microtubules ( ) . | The C-terminal region, also known as the interaction domain, is known to sequester tubulin heterodimers. This region is comprised of a coiled-coil alpha helix. This region of stathmin is known to bind to helix 10 of alpha tubulin. Helix 10 of alpha tubulin is thought to be important for incorporation into microtubules ( ) . | ||
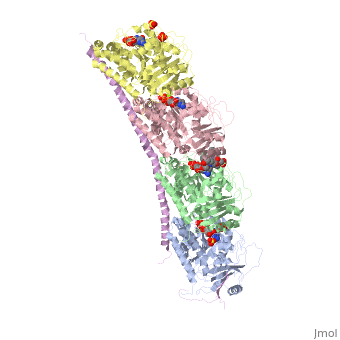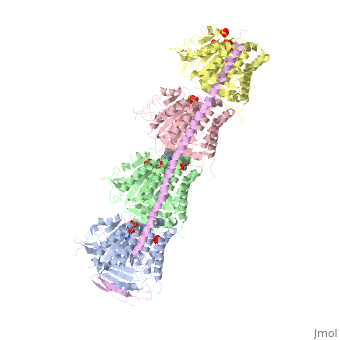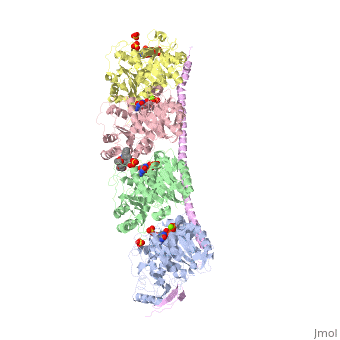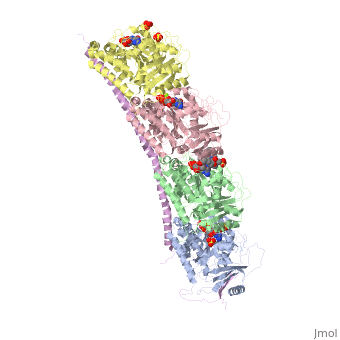
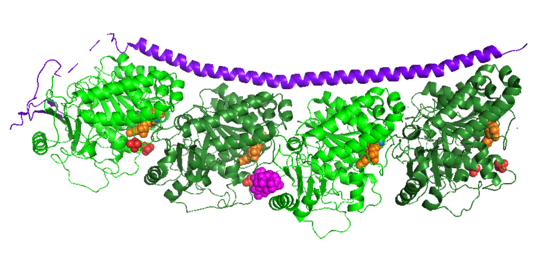
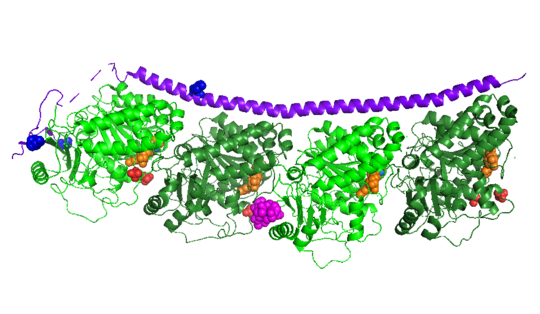
| - | The structure of stathmin, in 4eb6, is bound to two tubulin heterodimers. The tubulin dimers are bound to outside ligands. <scene name='77/778894/Vinblastine/1'>Vinblastine</scene> is a chemotherapeutic that binds to tubulin to prevent microtubule polymerization ( ) | + | The structure of stathmin, in 4eb6, is bound to two tubulin heterodimers. The tubulin dimers are bound to outside ligands. <scene name='77/778894/Vinblastine/1'>Vinblastine</scene> is a chemotherapeutic that binds to tubulin to prevent microtubule polymerization ( ). The tubulin subunits are bound to from <scene name='77/778894/Gtp_gdp_highlight_of_tubulin/1'>GTP/GDP.</scene> The beta subunits of tubulin are bound to GDP and each of the alpha subunits are bound to GTP and a Magnesium ion. There are also <scene name='77/778894/Mutations_in_stathmin/1'>two mutations</scene> to the stathmin in this structure. The mutations at position 11 from an cysteine to an alanine and position 16 from a Phenylalanine to a Tryptophan. |
[[Image:Stathmin_mutations.png|center|thumb| upright=3| Figure XXX. ]] | [[Image:Stathmin_mutations.png|center|thumb| upright=3| Figure XXX. ]] | ||
Revision as of 18:30, 27 April 2018
* Full Real Name: Alisa Cario
- Position: Graduate Student
- Institution (NO ABBREVIATIONS): University of Vermont
- City, State/Province, Country: Burlington, VT USA
- Field of Expertise or Study: Creation of protopedia page for a class project. The class is Proteins 1 under Dr. Stephen Everse
Stathmin-4 (RB3) bound to Tubulin stabilized with Vinblastin
4eb6
| |||||||||||