Garman lab: Interconversion of lysosomal enzyme specificities
From Proteopedia
(→Structures shown on this page) |
(→Structures shown on this page) |
||
| Line 58: | Line 58: | ||
<jmol> | <jmol> | ||
<jmolButton> | <jmolButton> | ||
| - | <script> | + | <script> hide none</script> |
<text>show GalNAc model</text> | <text>show GalNAc model</text> | ||
</jmolButton> | </jmolButton> | ||
Revision as of 18:59, 1 May 2018
Contents |
About this page
Lysosomal storage disease
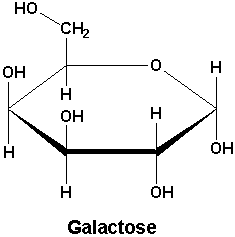
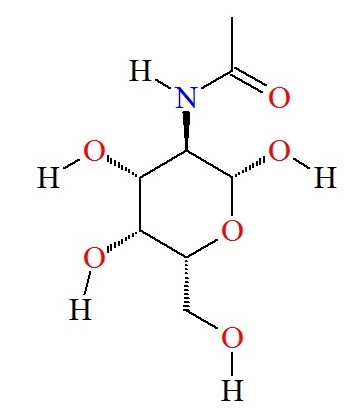
Lysosomal storage disorders are inherited metabolic diseases characterized by an accumulation of undigested various toxic materials. There are nearly 50 diseases and the two examples shown here are Fabry and Schindler disease. Fabry disease, which occurs between early childhood and adolescence, is characterized by the lack of the enzyme alpha galactosidase (α-Gal). Schindler disease can occur in infancy or in adulthood and is characterized by the lack on the enzyme alpha N-acetylgalactosaminidase (α-NaGal). There are currently no cures for lysosomal storage disorders however enzyme replacement therapy is a treatment option. The basic principle of enzyme replacement therapy is to over express the enzyme of interest heterologously, in this case α-Gal α-NaGal, in a cell line and to isolate and purify it from the culture. In enzyme replacement therapy, patients are injected with the enzymes that they lack in the hopes of restoring the enzymatic activity in their cells.
Immune Response
Individuals suffering from Fabry disease cannot produce the Alpha-Gal protein that is necessary for breaking down Galactose. The usual treatment for this is giving the patient doses of the protein, but this poses a problem. Since the body does not produce the protein, an immune response ranging from severe anaphylaxis to mild discomfort can occur when the patient is given the protein. The body does however produce Alpha NAGAL, a protein with a similar active site and function as Alpha Gal. Altering the active site of Alpha NAGAL to match that of Alpha Gal allows doctors to administer a protein that serves the function of Alpha Gal but has the antigenicity of Alpha NAGAL, which means the body will recognize the protein and not elicit an immune response.
Enzymatic activity
α-Gal and α-NaGal have relatively identical active sites, which are conserved with the exception of alanine, serine, glutamate and leucine which are positioned differently. The two enzymes have the same folds and both function by cleaving glycosydic bonds however have different substrate specificities. The differences in substrate specificity occur because α-NaGal has a larger binding pocket thus interacting with larger molecules but smaller residues.
Structures shown on this page
3H54: alpha-NAGAL with GalNAc
3HG5: alpha-GAL with Gal
3LX9: alpha-GAL(SA) in complex with GalNAc
3LXA: alpha-GAL(SA) in complex with Galactose
3LXC: alpha-GAL(SA) in presence of Glycerol
| |||||||||||