We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Collagen Structure & Function
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 14: | Line 14: | ||
These three α-chains are then twisted around one another in a rope-like manner to produce the overall tightly packed triple-helical form of the molecule. The interaction of α-chains is stabilized via interchain hydrogen bonding making the molecule fairly resistant to attack by other molcules. Each α-chain is surrounded by a hydration sphere which allows a hydrogen bonding network to be present between the water molecules and the peptide acceptor groups.<ref name="collalike" />. This hydrogen bonding occurs when the amino group (NH) of a glycine residue forms a peptide bond with the carbonyl (C=0) of an adjacent residue. The overall molecule is approxiametly 300nm long and 1.5-2nm in diameter.<ref name="collalike" />. | These three α-chains are then twisted around one another in a rope-like manner to produce the overall tightly packed triple-helical form of the molecule. The interaction of α-chains is stabilized via interchain hydrogen bonding making the molecule fairly resistant to attack by other molcules. Each α-chain is surrounded by a hydration sphere which allows a hydrogen bonding network to be present between the water molecules and the peptide acceptor groups.<ref name="collalike" />. This hydrogen bonding occurs when the amino group (NH) of a glycine residue forms a peptide bond with the carbonyl (C=0) of an adjacent residue. The overall molecule is approxiametly 300nm long and 1.5-2nm in diameter.<ref name="collalike" />. | ||
| - | The image on the right-hand side has each side chain colored a different color to shown how each individual <scene name='Sandbox_168/Helices/1'> | + | The image on the right-hand side has each side chain colored a different color to shown how each individual <scene name='Sandbox_168/Helices/1'>helices</scene> interact with the others to form the overall molecule. The <scene name='Sandbox_168/Myscene/1'>active sites</scene> |
have also been illustrated to point out their positions in the triple-helix. | have also been illustrated to point out their positions in the triple-helix. | ||
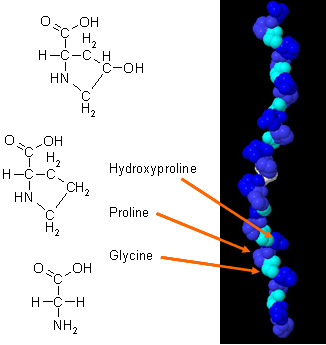
| - | [[Image:collagen_(alpha_chain).jpg | thumb |'''Figure 1.''' Amino Acid residues in collagen. Gly, Pro and Hydroxyproline residues present in a collagen molecule <ref name="residues"/>.]] | + | [[Image:collagen_(alpha_chain).jpg |400px| thumb |'''Figure 1.''' Amino Acid residues in collagen. Gly, Pro and Hydroxyproline residues present in a collagen molecule <ref name="residues"/>.]] |
| - | + | {{Clear}} | |
==Function== | ==Function== | ||
There are close to 30 different types of collagen that have been identified so far.<ref name="types">PMID:17581806</ref>. | There are close to 30 different types of collagen that have been identified so far.<ref name="types">PMID:17581806</ref>. | ||
| Line 41: | Line 41: | ||
*Atopic Dermatitis (III) | *Atopic Dermatitis (III) | ||
| - | + | </StructureSection> | |
==References== | ==References== | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 PMID:PMC1367617
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Bella J, Eaton M, Brodsky B, Berman HM. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science. 1994 Oct 7;266(5182):75-81. PMID:7695699
- ↑ 3.0 3.1 Yamazaki CM, Kadoya Y, Hozumi K, Okano-Kosugi H, Asada S, Kitagawa K, Nomizu M, Koide T. A collagen-mimetic triple helical supramolecule that evokes integrin-dependent cell responses. Biomaterials. 2010 Mar;31(7):1925-34. Epub 2009 Oct 22. PMID:19853297 doi:10.1016/j.biomaterials.2009.10.014
- ↑ Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929-58. PMID:19344236 doi:10.1146/annurev.biochem.77.032207.120833
- ↑ 5.0 5.1 Koide T. Designed triple-helical peptides as tools for collagen biochemistry and matrix engineering. Philos Trans R Soc Lond B Biol Sci. 2007 Aug 29;362(1484):1281-91. PMID:17581806 doi:10.1098/rstb.2007.2115
Proteopedia Page Contributors and Editors (what is this?)
Daman K. Kandola, Alexander Berchansky, David Canner, Andrea Gorrell, Luis Netto