We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
EPSP synthase
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Structural insights == | == Structural insights == | ||
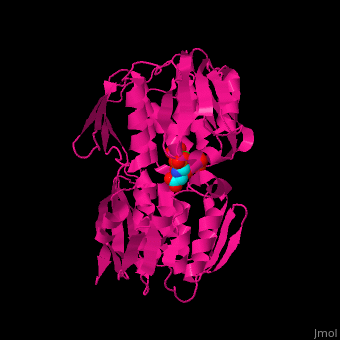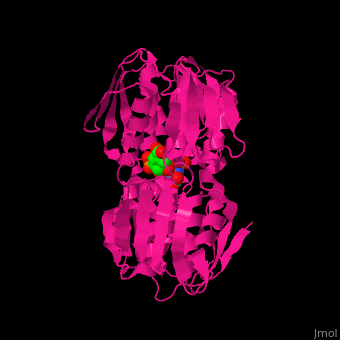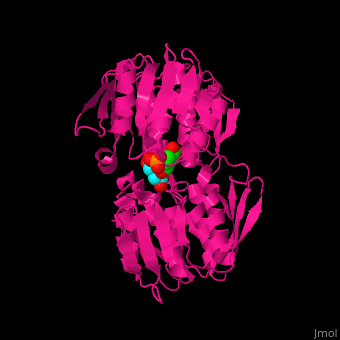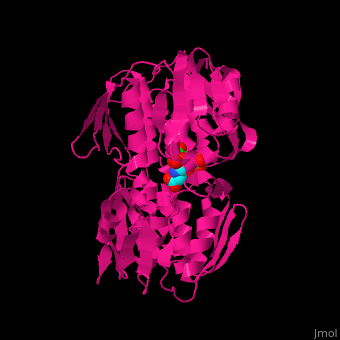
| - | The enzyme has two domains, with the active site found in the interdomain cleft <scene name='57/570585/Two_domains/2'>(open conformation)</scene>. There is a substantial structural change upon substrate binding, resulting in a <scene name='57/570585/Closed_formation/2'>closed</scene> conformation. <scene name='57/570585/Cv/3'>See animation of this process</scene>. '''Glyphosate''' (also known as '''Roundup''') occupies the of the second substrate, phosphoenol pyruvate <ref>PMID:11171958</ref>. Interestingly CP4 EPSP synthase still binds glyphosate in the absence of PEP, but a conformational change in glyphosate to accomodate a steric clash with <scene name='57/570585/Glyphosate_s3p_distance/2'>Glu 354</scene> changes the IC50 by a factor of over 4,000, from 2.5 micromolar to 11 millimolar. | + | The enzyme has two domains, with the active site found in the interdomain cleft <scene name='57/570585/Two_domains/2'>(open conformation)</scene>. There is a substantial structural change upon substrate binding, resulting in a <scene name='57/570585/Closed_formation/2'>closed</scene> conformation. <scene name='57/570585/Cv/3'>See animation of this process</scene>. '''Glyphosate''' (also known as '''Roundup''') occupies the of the second substrate, phosphoenol pyruvate <ref>PMID:11171958</ref>. Interestingly CP4 EPSP synthase still binds glyphosate in the absence of PEP, but a conformational change in glyphosate to accomodate a steric clash with <scene name='57/570585/Glyphosate_s3p_distance/2'>Glu 354</scene> shortens the length of glyphosate, from 7.3 angstroms to 6.67 angstroms, and changes the IC50 by a factor of over 4,000, from 2.5 micromolar to 11 millimolar. |
</StructureSection> | </StructureSection> | ||
Revision as of 15:18, 14 August 2018
| |||||||||||
3D structures of EPSP synthase
Updated on 14-August-2018
References
- ↑ Priestman MA, Healy ML, Funke T, Becker A, Schonbrunn E. Molecular basis for the glyphosate-insensitivity of the reaction of 5-enolpyruvylshikimate 3-phosphate synthase with shikimate. FEBS Lett. 2005 Oct 24;579(25):5773-80. PMID:16225867 doi:10.1016/j.febslet.2005.09.066
- ↑ Funke T, Han H, Healy-Fried ML, Fischer M, Schonbrunn E. Molecular basis for the herbicide resistance of Roundup Ready crops. Proc Natl Acad Sci U S A. 2006 Aug 29;103(35):13010-5. Epub 2006 Aug 17. PMID:16916934
- ↑ Schonbrunn E, Eschenburg S, Shuttleworth WA, Schloss JV, Amrhein N, Evans JN, Kabsch W. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1376-80. PMID:11171958 doi:http://dx.doi.org/10.1073/pnas.98.4.1376
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Ann Taylor, Joel L. Sussman