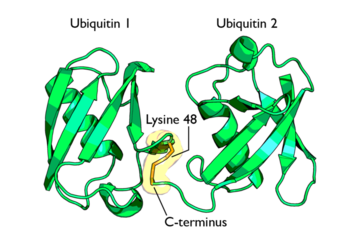We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ubiquitin Structure & Function
From Proteopedia
(Difference between revisions)
(→Diseases) |
|||
| Line 1: | Line 1: | ||
| + | ==Your Heading Here (maybe something like 'Structure')== | ||
| + | <StructureSection load='3rec' size='350' side='right' caption='Escherichia coli reca protein-bound DNA (PDB entry [[3rec]])' scene=''> | ||
| + | |||
| + | Anything in this section will appear adjacent to the 3D structure and will be scrollable. | ||
| + | |||
| + | |||
| + | |||
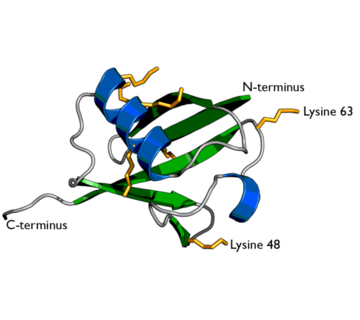
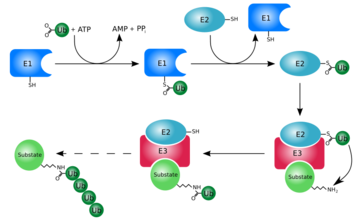
[[Ubiquitin]] is a single 8565 M<sub>r</sub> polypeptide consisting of 76 amino acid residues. Ubiquitin is highly known for its role in ATP-dependent protein degradation<ref name="mainpaper">PMID: 3041007</ref> | [[Ubiquitin]] is a single 8565 M<sub>r</sub> polypeptide consisting of 76 amino acid residues. Ubiquitin is highly known for its role in ATP-dependent protein degradation<ref name="mainpaper">PMID: 3041007</ref> | ||
{{STRUCTURE_1ubq| PDB=1ubq | SIZE=400| SCENE= |right|CAPTION=Human ubiquitin, [[1ubq]] }} | {{STRUCTURE_1ubq| PDB=1ubq | SIZE=400| SCENE= |right|CAPTION=Human ubiquitin, [[1ubq]] }} | ||
| Line 38: | Line 45: | ||
Infectious agents can manipulate ubiquitin or deubiquitination and one such protein is Chlamydia trachomatis. Chlamydia trachomatis' protein Cdu-1 catalyzes the hydrolysis of ubiquitin chains from Mcl-1. When polyubiquitnated, Mcl-1 is destined to be degraded by the proteasome, lowering the level of Mcl-1 and subsequently leading to apoptosis. The activity of Cdu-1 counteracts this by removing the ubiquitin, thus leading to higher levels of Mcl-1 in the cell. Additional information can be found here [[User:Karsten Theis/5B5Q]] | Infectious agents can manipulate ubiquitin or deubiquitination and one such protein is Chlamydia trachomatis. Chlamydia trachomatis' protein Cdu-1 catalyzes the hydrolysis of ubiquitin chains from Mcl-1. When polyubiquitnated, Mcl-1 is destined to be degraded by the proteasome, lowering the level of Mcl-1 and subsequently leading to apoptosis. The activity of Cdu-1 counteracts this by removing the ubiquitin, thus leading to higher levels of Mcl-1 in the cell. Additional information can be found here [[User:Karsten Theis/5B5Q]] | ||
| + | </StructureSection> | ||
==3D structures of ubiqitin== | ==3D structures of ubiqitin== | ||
Revision as of 06:18, 30 August 2018
Your Heading Here (maybe something like 'Structure')
| |||||||||||
3D structures of ubiqitin
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Vijay-Kumar S, Bugg CE, Cook WJ. Structure of ubiquitin refined at 1.8 A resolution. J Mol Biol. 1987 Apr 5;194(3):531-44. PMID:3041007
- ↑ 2.0 2.1 Vijay-Kumar S, Bugg CE, Wilkinson KD, Cook WJ. Three-dimensional structure of ubiquitin at 2.8 A resolution. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3582-5. PMID:2987935
- ↑ Cox MJ, Haas AL, Wilkinson KD. Role of ubiquitin conformations in the specificity of protein degradation: iodinated derivatives with altered conformations and activities. Arch Biochem Biophys. 1986 Nov 1;250(2):400-9. PMID:3022650
- ↑ Wilkinson KD. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J. 1997 Dec;11(14):1245-56. PMID:9409543
- ↑ Hochstrasser, M. 1996. Ubiquitin-dependent protein Degradation. Annu Rev Genet. 30: 405-439
- ↑ Snyder PM, Price MP, McDonald FJ, Adams CM, Volk KA, Zeiher BG, Stokes JB, Welsh MJ. Mechanism by which Liddle's syndrome mutations increase activity of a human epithelial Na+ channel. Cell. 1995 Dec 15;83(6):969-78. PMID:8521520
Proteopedia Page Contributors and Editors (what is this?)
Jaclyn Gordon, Joel L. Sussman, Michal Harel, David Canner, Andrea Gorrell, Alexander Berchansky, Karsten Theis